Authors: Aditya Mandawat, MD; Denise Garman, BS, RT; Denise Morell, RT; Stephen Darty, RT; Clara Cheek, RN; Tina D. Tailor, MD
Institute: Duke Cardiovascular Magnetic Resonance Center, Duke University, Durham, NC, USA
Clinical Presentation
A 59-year-old female with suspected dilated cardiomyopathy and no valvular pathology was referred for cardiac MRI. Resting EKG showed normal sinus rhythm, left ventricular hypertrophy, and non-specific T wave abnormalities.
CMR findings
Cine images of the right-ventricular outflow tract (Video 1) and pulmonary valve (Videos 2, 3) revealed an incidental hypointense mass on the pulmonic valve, measuring 8 x 7 mm. The mass was attached to the ventricular side of the right pulmonary valve cusp.
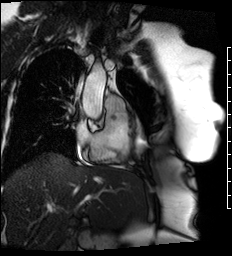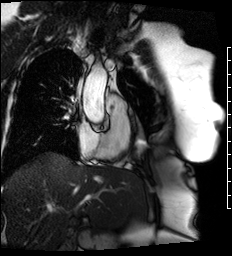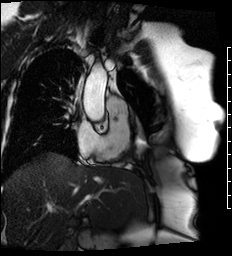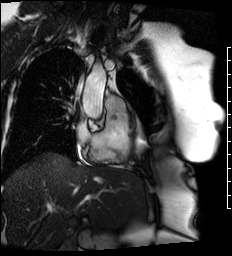
Video 1. Cine of the right-ventricular outflow tract demonstrating a mass attached to the ventricular side of the pulmonary valve.
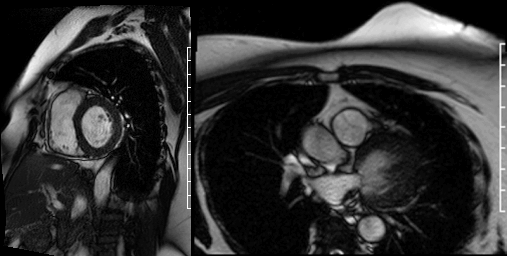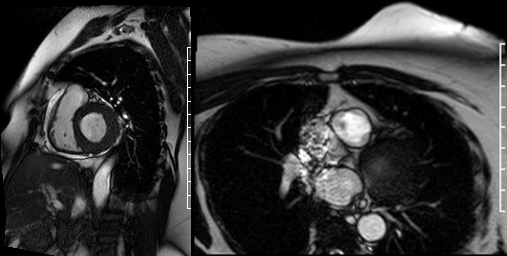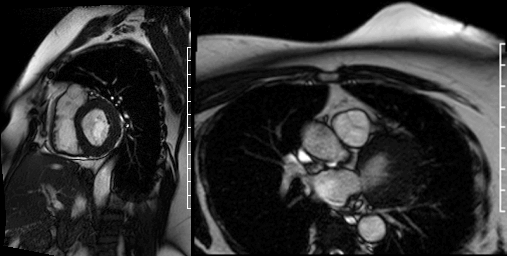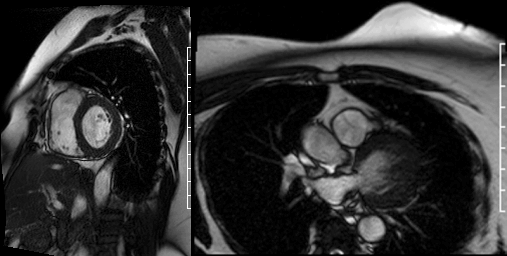
Videos 2 and 3. Axial and en face cine’s at the level of the pulmonary valve demonstrating a mass attached to the right cusp of the pulmonary valve.
Delayed gadolinium enhancement (DGE) imaging with a prolonged inversion time (TI = 600ms) demonstrated that the mass was indistinguishable from the blood pool, suggesting against the possibility of a valvular thrombus. (Figure 1) Black-blood DGE imaging with segmented inversion recovery gradient-echo technique demonstrated contrast enhancement throughout the mass. (Figure 2)
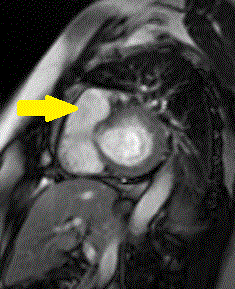
Figure 1. Long axis view of the right ventricle outflow tract using DGE imaging with prolonged inversion time (TI = 600ms) demonstrating that the pulmonic valve mass is indistinguishable from the blood pool (arrow).
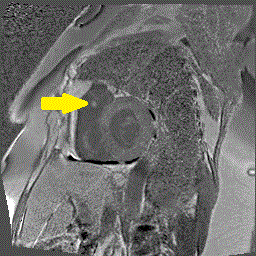
Figure 2. Dark blood DGE imaging in an axial plane, at the level of the pulmonary valve, demonstrating contrast enhancement in the pulmonic valve mass (arrow).
Conclusion
CMR findings of the pulmonary valve mass were most consistent with the diagnosis of a pulmonary valve papillary fibroelastoma (PFE).
Perspective
Contrast-enhanced CMR permits accurate differentiation of thrombus and cardiac tumors. On DGE utilizing inversion recovery technique with a prolonged inversion time, thrombus will appear hypointense (i.e. black) compared to the cardiac blood pool, whereas a cardiac tumor will appear isointense to the blood pool (i.e. grey). (1) We used a novel, dark-blood technique (FIDDLE) (2) to improve contrast between the pulmonary valve mass and the adjacent blood pool. While validated only in the imaging of myocardial infarction, this technique may be useful in the imaging of cardiac masses, particularly when delineation of the mass and the adjacent blood pool is poor.
PFE’s represent 70%-80% of heart valve tumors. (3) Characteristics features of PFE’s include: (a) attachment to the cardiac valves; (b) round, oval, or irregular shape with well-demarcated margins and homogenous texture; (c) small size (99% are less than 20 mm in largest dimension); and (d) small mobile stalks. (4)
The majority of PFE’s arise from the aortic side of the aortic valve. (4,5) While rarely causing valvular dysfunction, aortic PFE’s have been associated with dynamic coronary ostia obstruction, leading to myocardial ischemia, as well as transient ischemic attacks and stoke. (5) The mitral and tricuspid valves are the second most common sites of involvement. When located in the atrioventricular valves, the tumor most often occurs on the atrial side of the valve. (4,5) Pulmonary valve PFE’s are rare, with fewer than 10 cases reported in the literature. (5)
Considerable debate exists regarding the optimal management of PFE’s, particularly in asymptomatic patients. In a recent, large case series from the Mayo Clinic, Tamin and colleagues compared patients receiving cardiac surgery to remove PFE’s to those managed expectantly. (6) The valve was preserved in 98% of patients undergoing cardiac surgery, and tumor recurrence occurred in only 1.6% of patients by 1.6 years. Follow-up stroke risk at 5 years was 8% in the post-operative group, versus 13% in patients managed expectantly. In patients managed expectantly, patients taking warfarin, aspirin, or clopidogrel had stroke risks similar to those not taking medication (p=0.39), suggesting that anticoagulation may not be necessary.
Click here to access entire CMR study on CloudCMR.
References
1. Weinsaft JW, Kim RJ, Ross M et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovascular imaging 2009;2:969-79.
2. Kim HW, Rehwald WG, Jenista ER et al. Dark-Blood Delayed Enhancement Cardiac Magnetic Resonance of Myocardial Infarction. JACC Cardiovasc Imaging. 2017 Dec 8. pii: S1936-878X(17)30989-0.
3. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. The Lancet Oncology 2005;6:219-28.
4. Sun JP, Asher CR, Yang XS et al. Clinical and Echocardiographic Characteristics of Papillary Fibroelastomas. A Retrospective and Prospective Study in 162 Patients. Circulation. 2001;103:2687-2693.
5. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. American Heart Journal 2003;146:404-410.
6. Tamin SS, Maleszewski JJ, Scott CG et al. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. Journal of the American College of Cardiology 2015;65:2420-2429.
Case Prepared by: M. Jay Campbell, MD, MHA; Editor of SCMR Case of the Week, Duke University







