Case from: David Kassop, Edward A. Hulten, Maureen Hood, Robert A. Liotta, Vincent B. Ho.
Institute: Walter Reed National Military Medical Center and Uniformed Services University of Health Sciences, Bethesda, MD.
Clinical history: A 62 year-old Caucasian man presented to the Cardiology Clinic for a routine perioperative evaluation prior to juxtarenal abdominal aortic aneurysm repair. He was otherwise in his usual state of health without abnormal symptoms. He has a history of coronary artery disease that presented as an acute myocardial infarction almost thirty years ago. However, he had no intervention and has remained in class 1 functional status with normal left ventricular ejection fraction on secondary preventive medications. His physical examination demonstrated normal vital signs and no significant abnormalities.
The baseline electrocardiogram (Figure 1) demonstrated normal sinus rhythm and non-specific ST-T wave abnormalities with an isolated q wave in lead III. He has no history of arrhythmia and no significant family medical history. A transthoracic echocardiogram was technically limited by acoustic windows and interpreted clinically as left ventricular ejection fraction of 55-60% with no regional wall motion abnormalities (Movie 1).
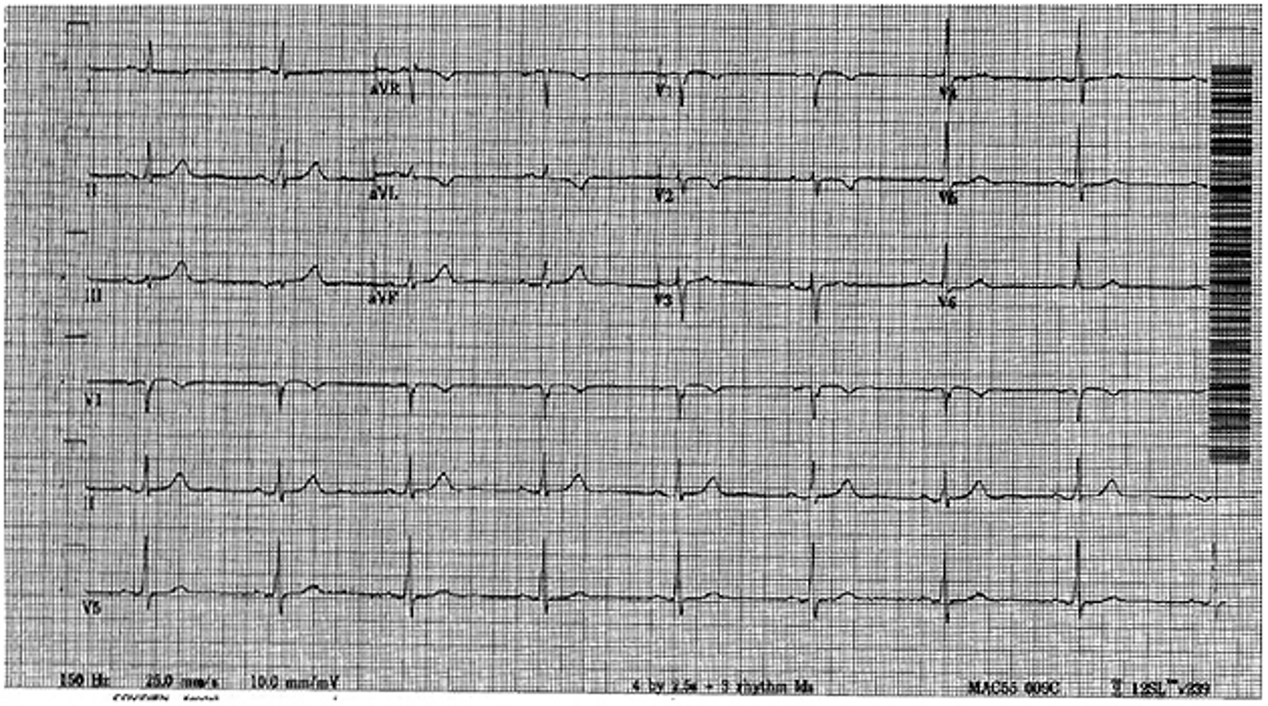
Figure 1. Baseline electrocardiogram demonstrating normal sinus rhythm and non-specific ST and T wave abnormalities. There is an isolated q wave in lead III but no electrocardiographic evidence of his prior left anterior descending territory infarction.
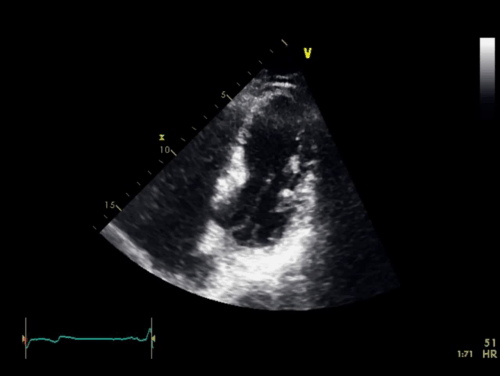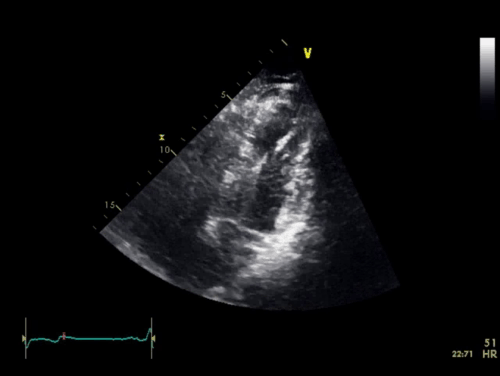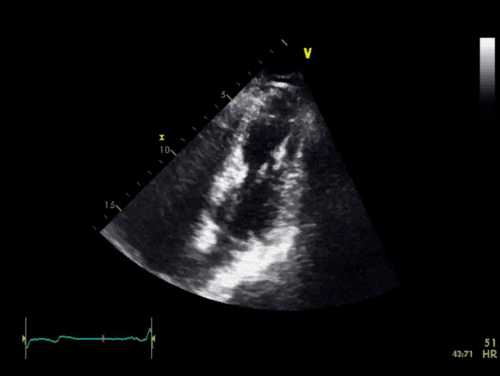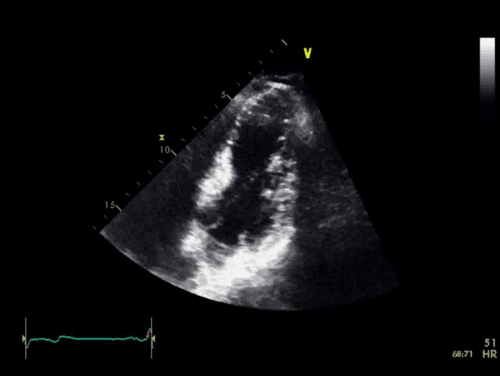
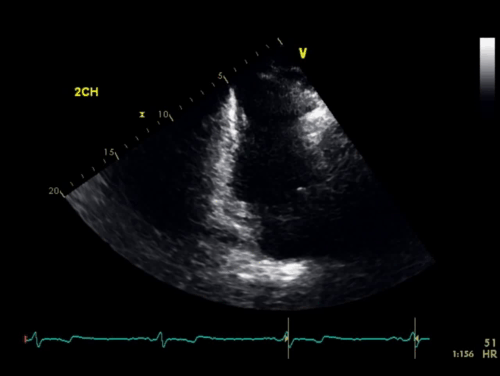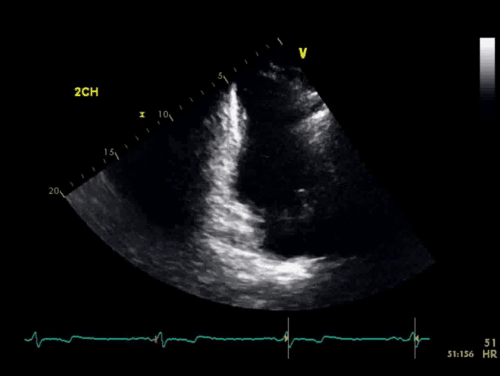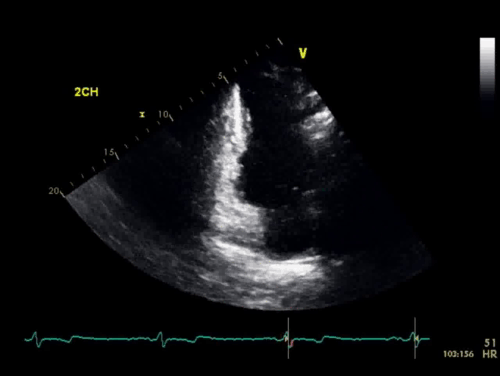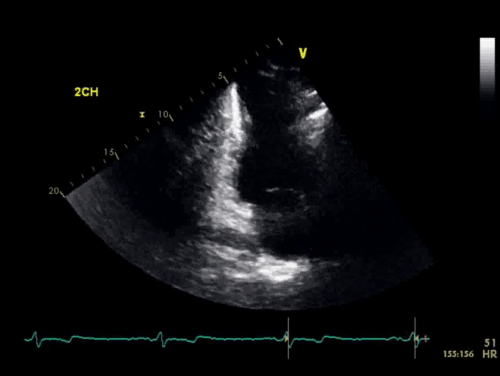
Movie 1. 4 chamber and 2 chamber echocardiogram, interpreted clinically as technically limited acoustic windows with left ventricular ejection fraction of 55-60% and no regional wall motion abnormalities.
A coronary computed tomography angiogram (CCTA) was performed (LightSpeed VCT; GE Healthcare, Waukesha, WI) for pre-operative risk assessment prior to elective high risk vascular surgery and demonstrated mild non-obstructive coronary artery disease in the proximal left anterior descending coronary artery (Figure 2).
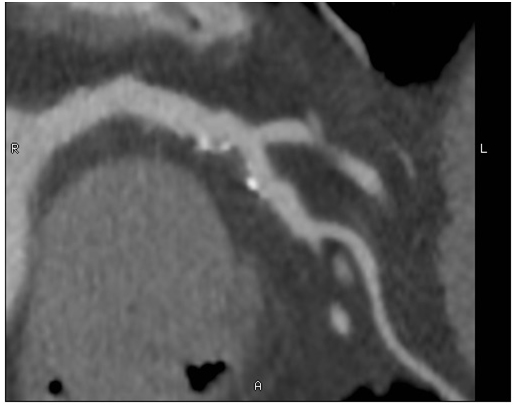
Figure 2. Coronary computed tomography angiogram of the left anterior descending coronary artery demonstrating a proximal predominantly non-calcified plaque with spotty calcifications, mild positive remodeling, and associated 25-50% stenosis.
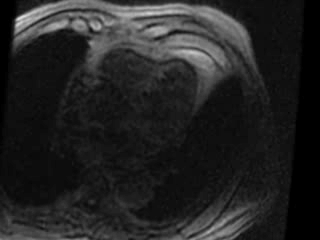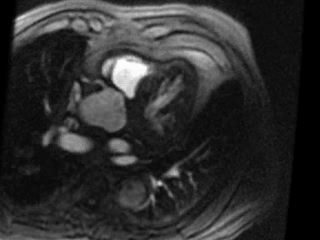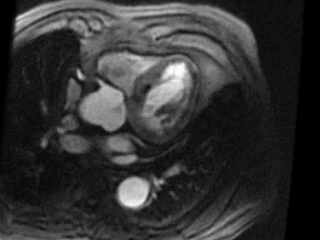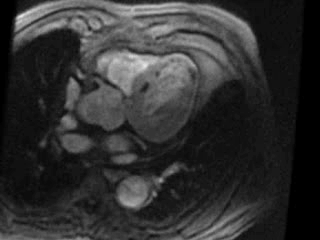
Incidentally noted was an extensive region (8 myocardial segments) of low Hounsfield Unit (HU) signal in the subendocardium from base to distal in the anterior and anteroseptum segment as well as apical lateral and the true apex. The signal (-80 HU) was consistent with fat (Figure 3).
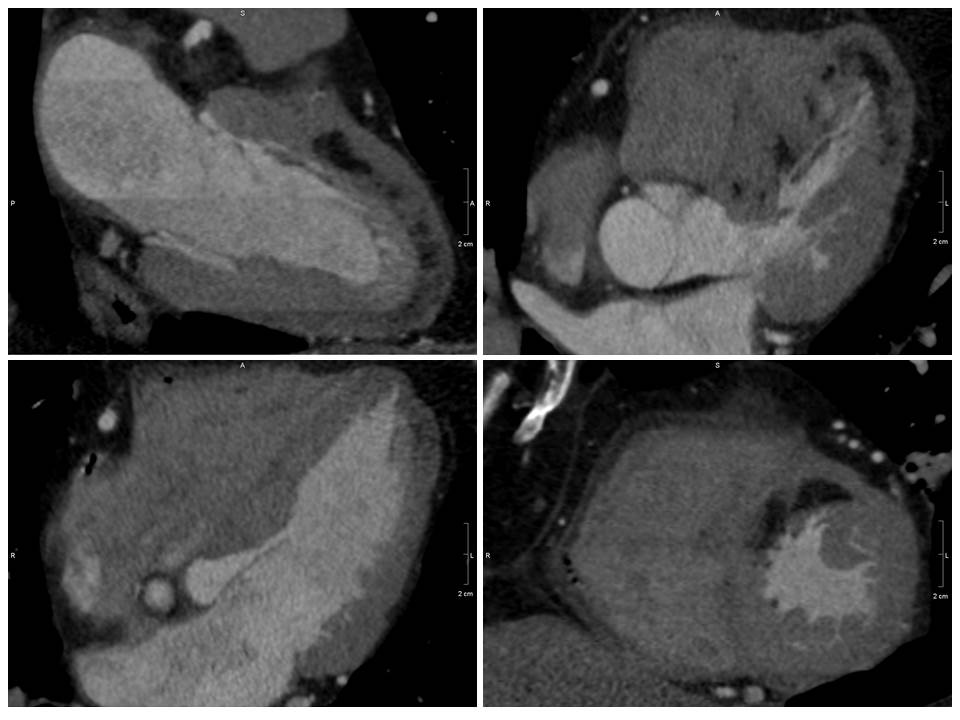
Figure 3. Coronary computed tomography demonstrating extensive (8 myocardial segments) low Hounsfield Unit signal (black region) consistent with fat in the distribution of a prior left anterior descending coronary artery infarct.
CMR Findings: A 1.5T (Signa HDxt, GE Healthcare, Waukeshau, WI, USA) cardiac magnetic resonance (CMR) study was obtained to further evaluate the extensive intra-myocardial fat. It demonstrated lobular mid-myocardial and subendocardial fatty infiltration in the same 8 myocardial segments of the left ventricular myocardium without associated myocardial wall thinning (Movie 2 and Figure 4). A first pass perfusion defect was noted in the affected area (Movie 3). There was patchy late gadolinium enhancement (LGE) within these areas (Figure 5), which did not have the typical appearance of infarct due to lipomatous metaplasia of scar.
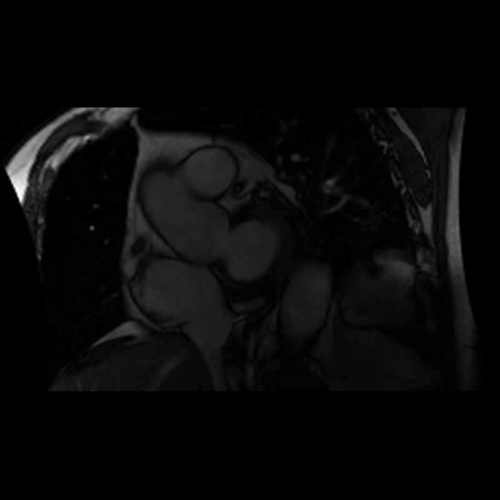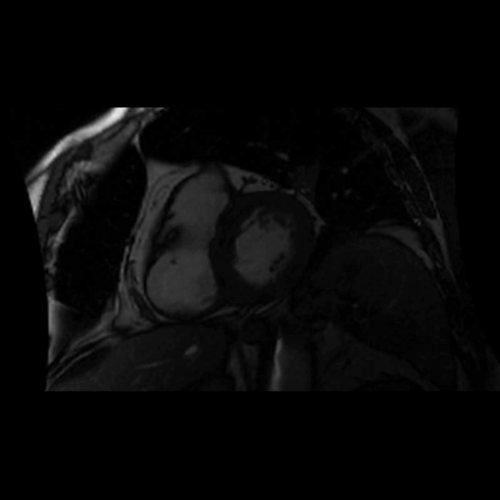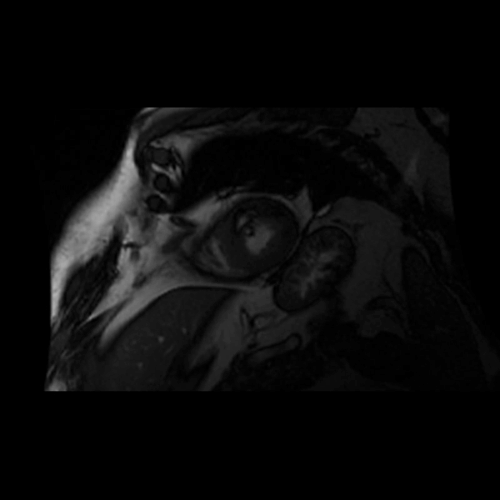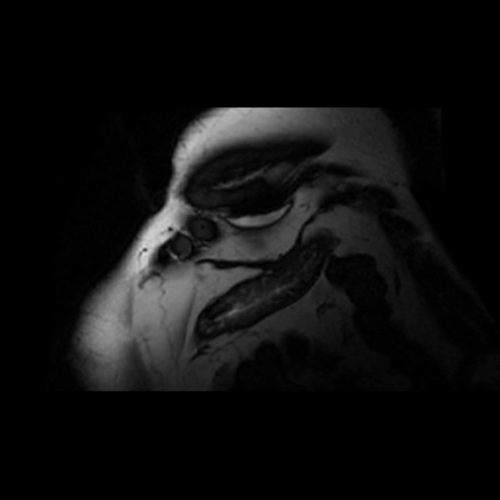
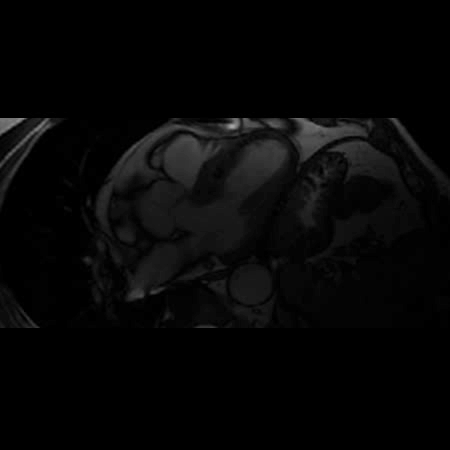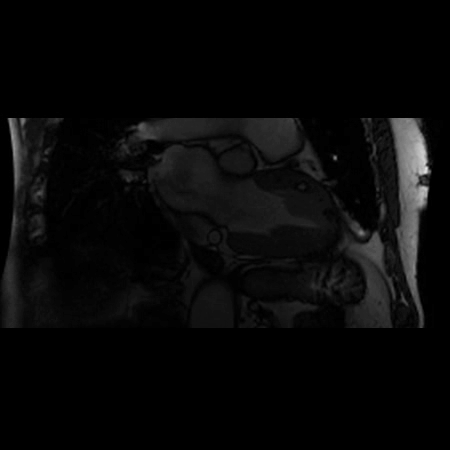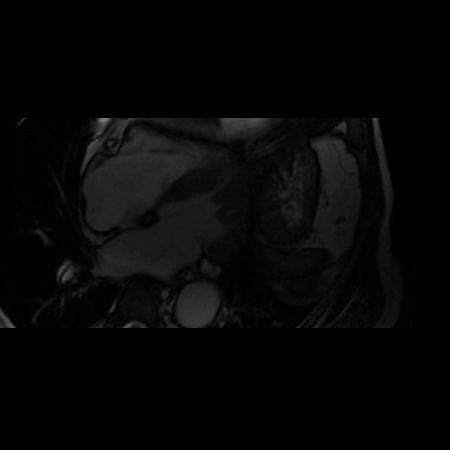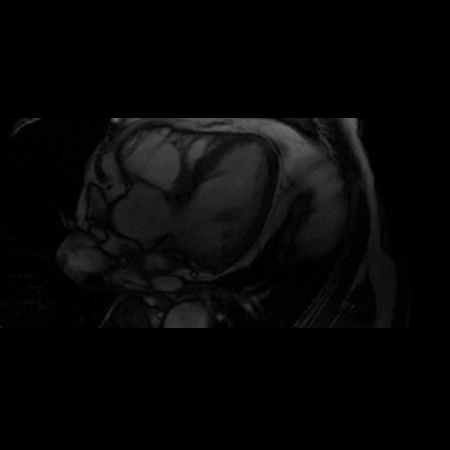
Movie 2. Balanced steady state free precession (SSFP) short axis stack and long axis cine demonstrating intra-myocardial signal hyperintense relative to the myocardium (consistant with fat).
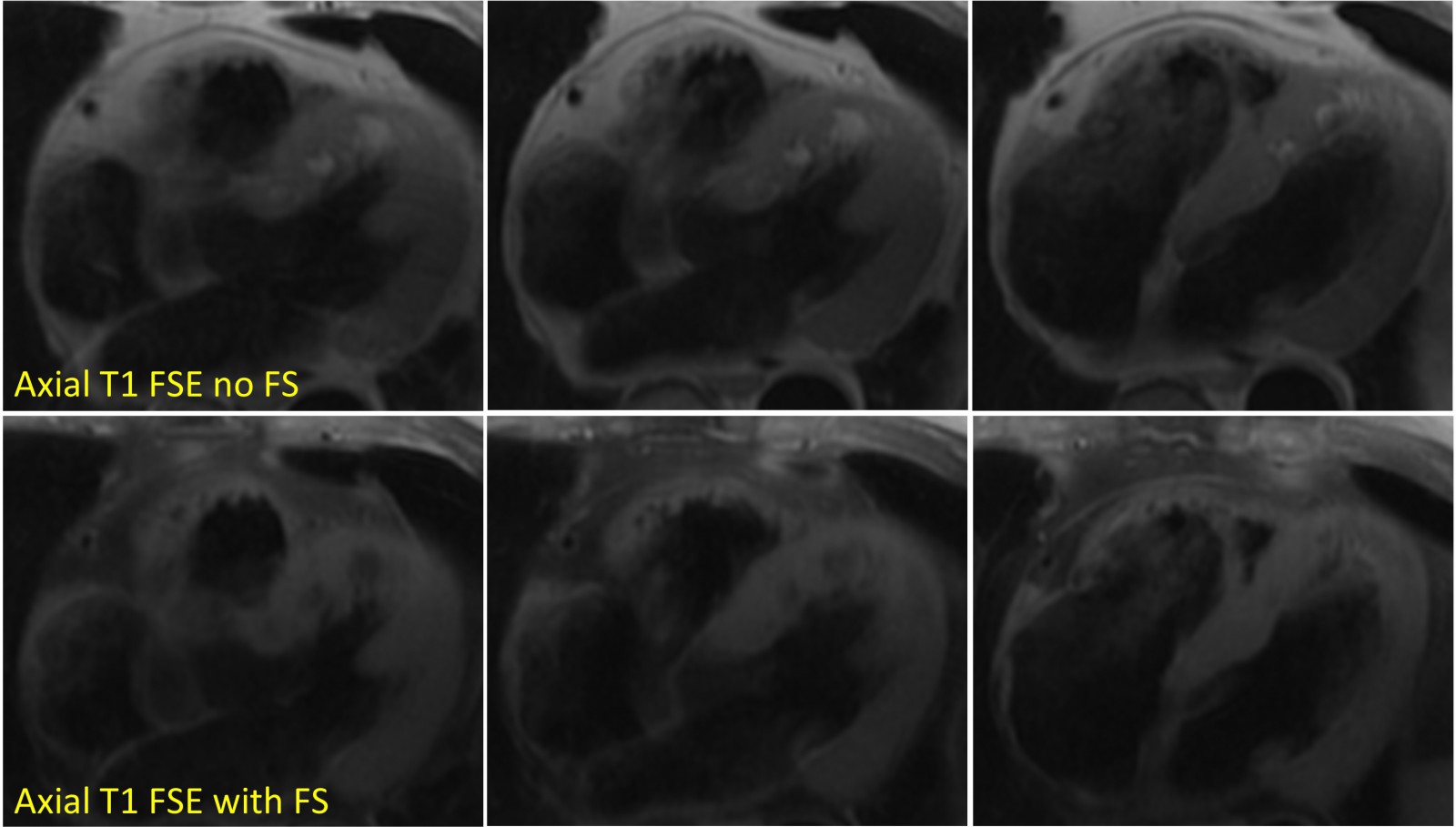
Figure 4. Axial T1-weighted fast spin echo (upper panel, without fat suppression) demonstrating high signal intensity in the anteroseptal wall from base to distal and the apical lateral wall. The signal appropriately suppresses with fat suppression (lower panel).
Movie 3. Axial view of gadolinium contrast enhanced first pass perfusion demonstrating a resting perfusion defect in the territory of the LAD.
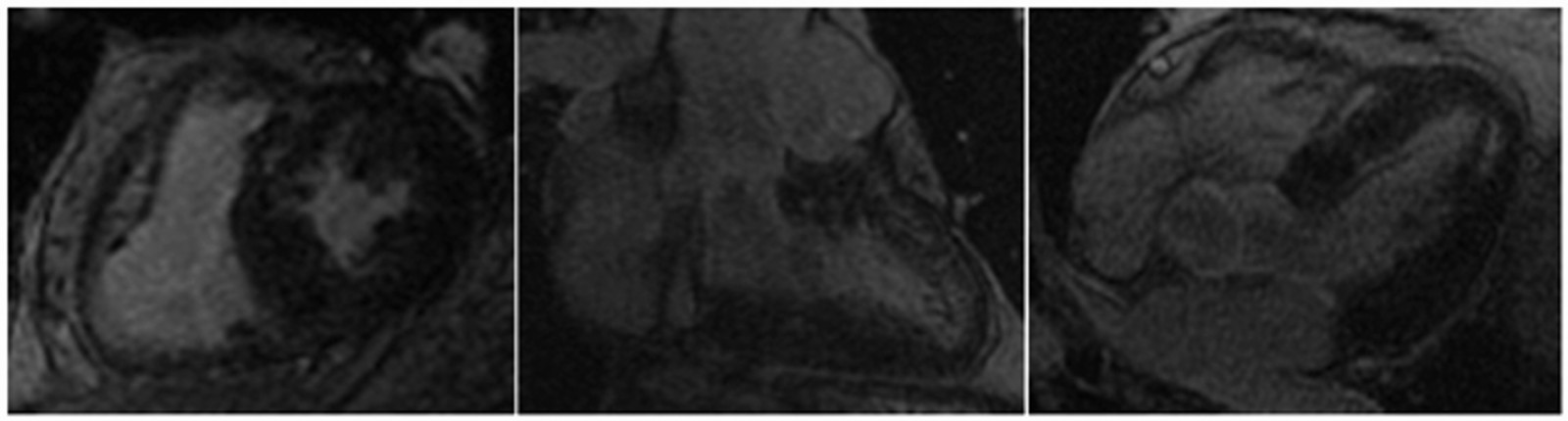
Figure 5. Late gadolinium enhancement demonstrating patchy fibrosis not in a typical infarct pattern due to lipomatous metaplasia.
Conclusion: Overall the findings were most consistent with lipomatous metaplasia of infarct in the LAD distribution, and the patient underwent aortic aneurysm repair without complication.
Perspective: At the time of CCTA, the extensive intra-myocardial fat was surprising given his remote history of infarction, excellent functional status, and consistently unremarkable ECG and echocardiography. As a first teaching point, this case illustrates the sub-optimal sensitivity of ECG or echocardiography for detection of infarction.1 Second, this case demonstrates how a comprehensive cardiac MRI can confidently characterize tissue characteristics of myocardial lesions. Given his extensive fatty involvement of the myocardium on CT, initial diagnostic consideration of liposarcoma or left sided arrhythmogenic cardiomyopathy were discussed as possible alternative, albeit less favorable, diagnoses. However, the patient had no clinical signs or symptoms of malignancy or arrhythmia to correlate with these diagnoses, illustrating the importance of clinical correlation with advanced imaging. Also, the area of involvement in a typical infarct territory of the LAD favors a diagnosis of lipomatous metaplasia. Fatty infiltration has also been reported in patients with tuberous sclerosis, although not clinically relevant in this case. Next, the case represents an uncommon presentation (extensive lipomatous metaplasia) of a common disease (myocardial infarction). Although lipomatous metaplasia of scar is common at a histologic level, extensive involvement of the myocardium has not been widely reported.2 From a histologic study of explanted hearts of patients undergoing heart transplantation with known ischemic cardiomyopathy, 141 of 168 (84%) of old MI had some lipomatous metaplasia, although only 22% of the lesions were composed of >25% fat.3 When patients with known prior MI are referred to CCTA, a 24% prevalence of lipomatous metaplasia has been reported, although the extent of involvement was not noted.4 Although lipomatous metaplasia has been commonly reported by CT, due to referral biases the prevalence and clinical significance are poorly characterized and studies have completely disparate conclusions regarding any association or not with age of infarct, serum cholesterol, adiposity, milder CAD, decreased contractility, or arrhythmia.3, 4 These areas of clinical significance warrant further study.
Disclosures. The opinions and assertions expressed herein are the authors’ alone and do not represent Walter Reed National Military Medical Center, the US Army, or the Department of Defense.
References:
- Schelbert EB, Cao JJ, Sigurdsson S & et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 308, 890–896 (2012).
- Lücke, C. et al. Prevalence and functional impact of lipomatous metaplasia in scar tissue following myocardial infarction evaluated by MRI. Eur. Radiol. 20, 2074–2083 (2010).
- Su, L., Siegel, J. E. & Fishbein, M. C. Adipose tissue in myocardial infarction. Cardiovasc. Pathol. 13, 98–102 (2004).
- Ahn, S. S. et al. CT Detection of Subendocardial Fat in Myocardial Infarction. Am. J. Roentgenol. 192, 532–537 (2009).
COTW handling editor: Edward A. Hulten, MD
Have your say: What do you think? Latest posts on this topic from the forum







