Case from: Frances Perez MD, Gyanendra Sharma MD, Jayanth H Keshavamurthy MD.
Institute: Georgia Regents University, Division of Cardiology and Department of Radiology, Augusta, GA.
Clinical history: An 85 year old male veteran was referred to our institution for further evaluation of an incidental large aneurysm in the region of a ductus diverticulum, seen on a CT chest 2 years prior. An MRI of the chest was performed without the use of intravenous contrast as the patient has a single kidney and it was considered best to avoid exposure to contrast.
CMR Findings: An MRA was performed using the 3D whole heart sequence. In addition, T1 weighted dark blood inversion recovery sequences (Figure 1) and SSFP sequences (Figure 2) were performed. The images demonstrated a large ductus diverticulum /aneurysm arising from the inferior aspect of the aortic arch measuring 4.3 x 3.7 cm in the expected location of the ductus diverticulum (Figures 3-5). This lesion was stable in size as per prior CT chest report. Because the patient was asymptomatic and the lesion had been stable for 2 years, it was left alone. The patent ductus arteriosus was nonexistent. There were no calcifications to suggest a post traumatic pseudo aneurysm.
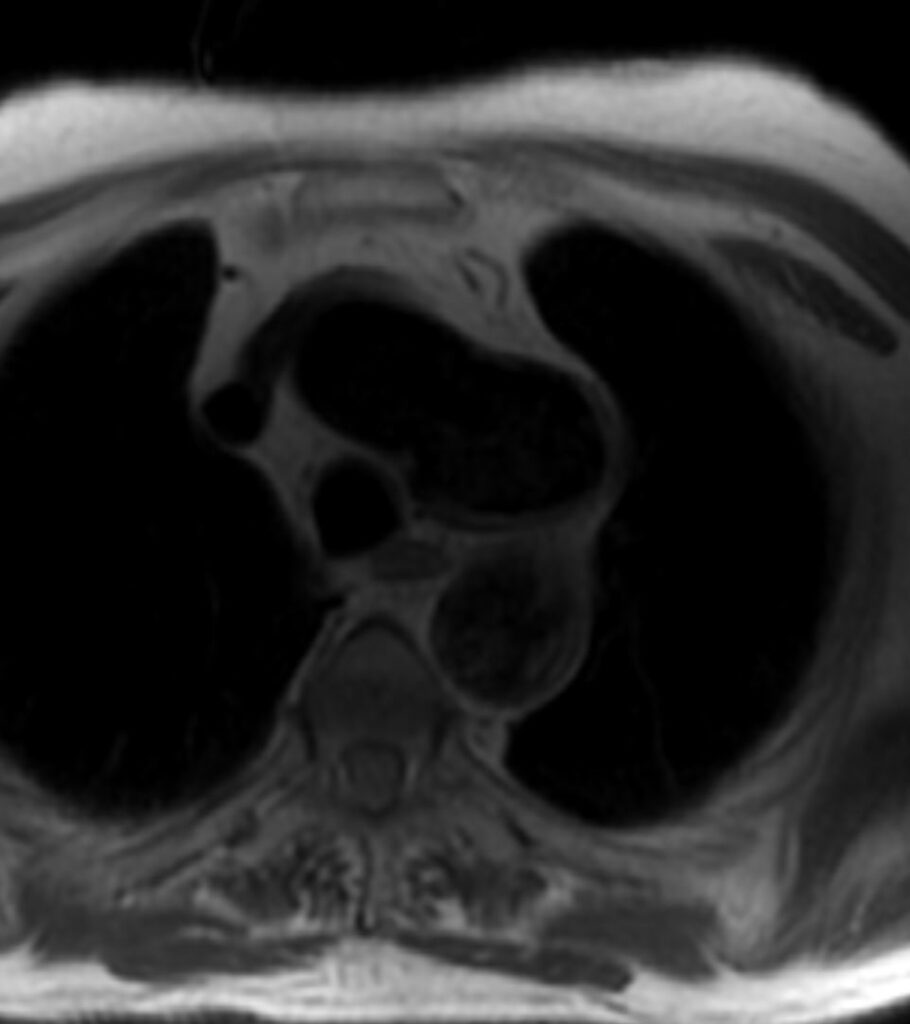
Figure 1. T1 weighted dark blood inversion recovery sequence
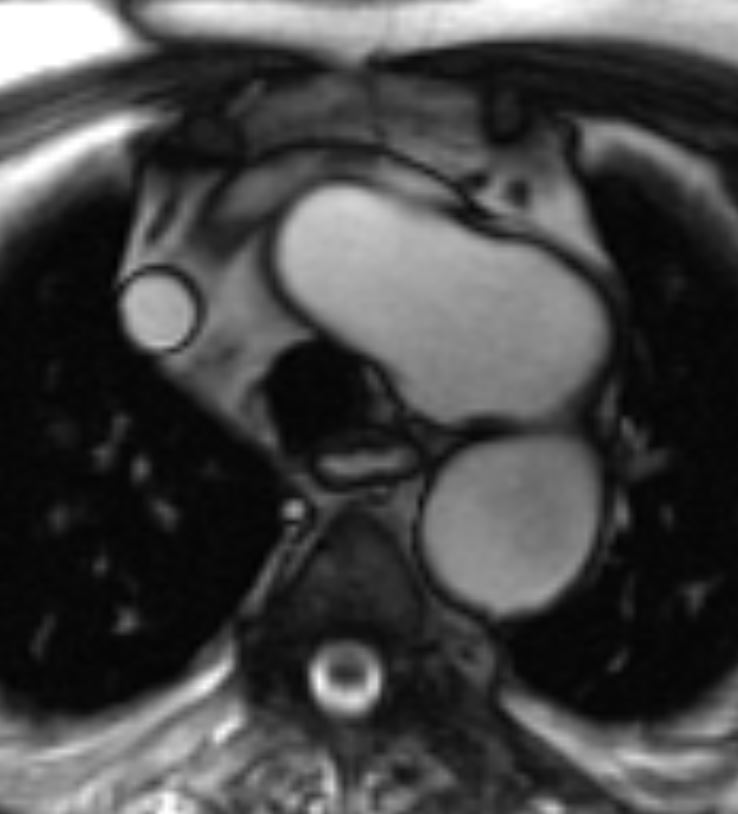
Figure 2. SSFP sequence
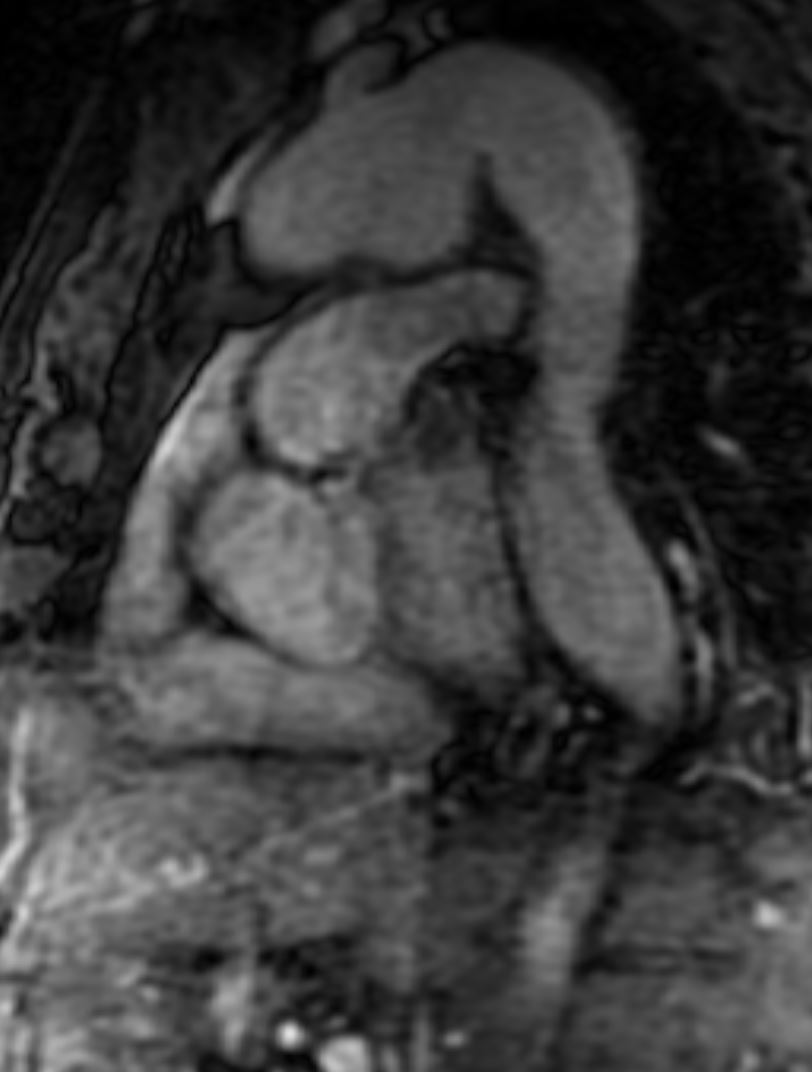
Figure 3. T1 weighted dark blood inversion recovery sequence
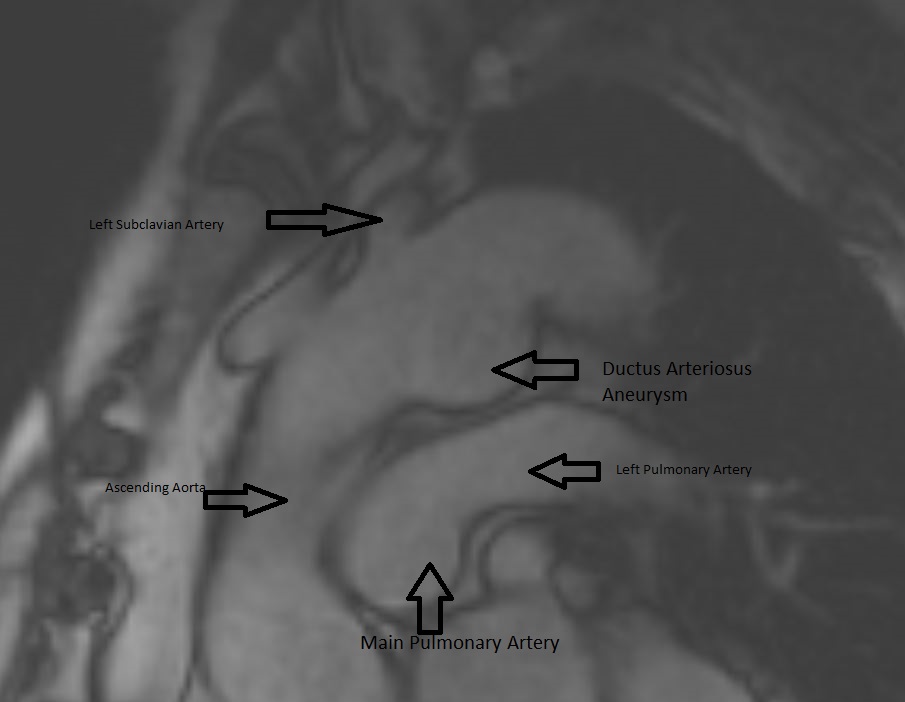
Figure 4. SSFP sequence
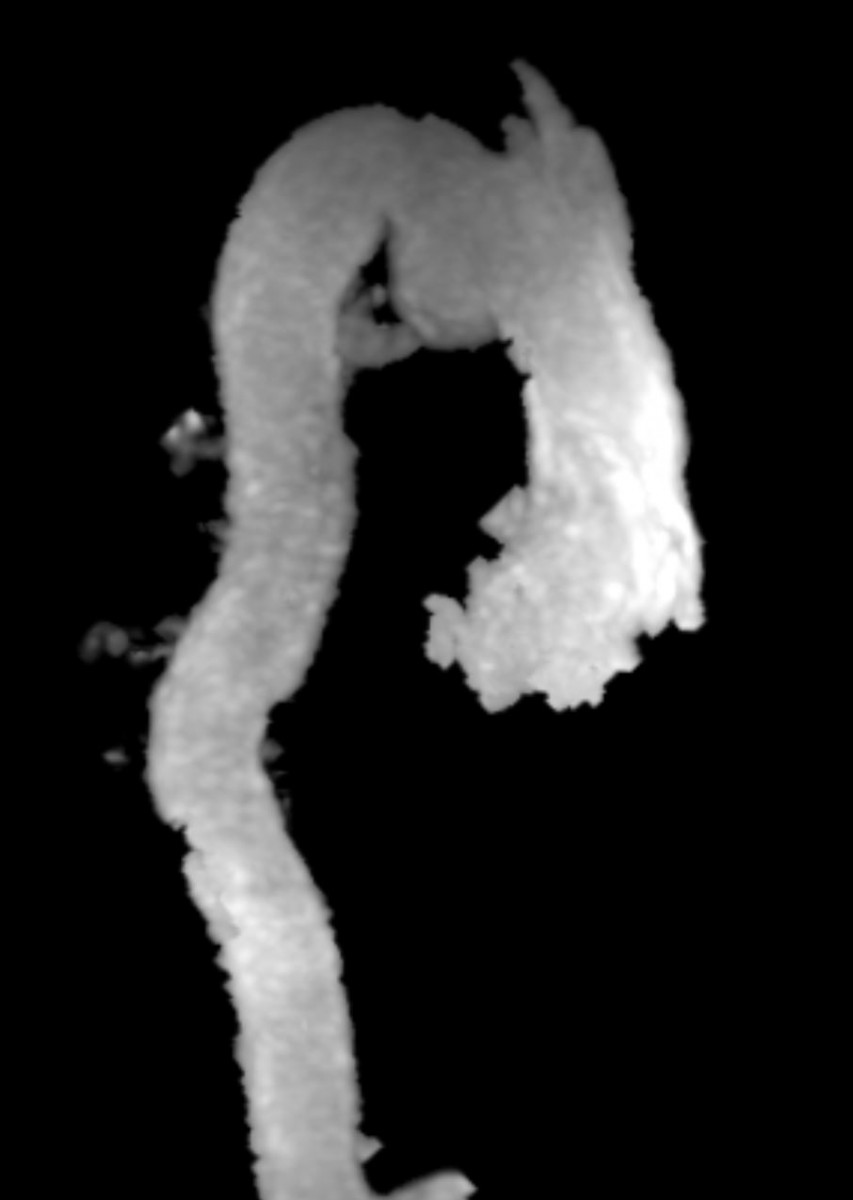
Figure 5. MRA can show the size, shape, and orientation of lesions with respect to the surrounding structures. No patent ductus arteriosus was noted.
Conclusion: Aneurysm of the ductus arteriosus is a rare entity found in adults, however, the recognition and ability to differentiate this from a traumatic aortic injury is crucial in order to prevent unnecessary surgical intervention. On CTA imaging, ductus arteriosus aneurysm would present as a luminal out-pouching in the aortopulmonary window, this is referred to as the “triple star” sign. MRA can non-invasively show the size, shape, and orientation of lesions with respect to the surrounding structures. Therefore, MRA is useful in determining if a patent ductus arteriosus is present, and in distinguishing a pseudoaneurysm from atypical ductus diverticulum. With our patient, we could determine from the MRA that there was no patent ductus arteriosus (Figure 5).
Perspective: The ductus arteriosus originates from the persistence of the distal portion of the left sixth aortic arch; it connects the descending aorta immediately distal to the left subclavian artery to the roof of the main pulmonary artery, near the origin of the left pulmonary artery. Persistence of the duct beyond 48 hours after birth is abnormal and results in patent ductus arteriosus (PDA). PDA is uncommon in adults as it is usually discovered and treated during childhood [1].The saccular or fusiform dilatation of the ductus arteriosus is called an aneurysm (DA), and aneurysm of the ductus arteriosus (DAA) is a rare condition in adults. Most of the ductus aneurysms arise from the non-patent ductus arteriosus with its pulmonary end obliterated and non-obliteration of ductus near the aortic arch end [2].
The pathogenesis of ductal aneurysm in the adult still remains uncertain. However, as all the ductal aneurysms arise from non-patent DA, incomplete obliteration of the DA is postulated. The non-patent DA initially becomes a ductus diverticulum, after being exposed to the high systemic aortic blood pressure the ductus arteriosus aneurysm develops.
The most commonly reported symptoms are non-specific such as cough, dyspnea and dysphonia. [3]. Laryngeal paralysis (Ortner’s syndrome) is an unusual presentation of DDA [4]. DAA can also present incidentally as a left-sided mediastinal mass, therefore prior to performing a lung biopsy of any left-sided mediastinal mass, it is essential to exclude vascular pathology. [3, 5, 6]. Extreme presentations of DAA include sudden rupture of aneurysm and death [7].
It is a diagnostic challenge for radiologist to distinguish a post traumatic aortic isthmus pseudoaneurysm from a normal type III ductus diverticulum on a trauma chest CTA, since both aortic entities occur in roughly the same anatomic location, often leading to diagnostic confusion. However, post-traumatic aneurysms of the thoracic aorta are typically located in the posterior part of the aortic arch just distal to the origin of the left subclavian trunk; and tend to grow towards the left. There are usually calcifications within the post traumatic pseudo aneurysms and there will likely also be a clinical history of trauma [3]. Additionally, one should also look for direct and indirect vascular signs of injury in the acute trauma cases [8]. It is crucial to differentiate posttraumatic injury from ductus diverticulum as former requires thoracotomy.
A ductus diverticulum can simulate an aortic dissection on axial images. Sagittal oblique reformatted images will show the smooth margins and the typical gentle obtuse angles formed by the ductus diverticulum with the aortic wall [9]. Goodman et al have described the distinct variations of ductus diverticulum [10]. It is hard to say whether our case is a large type 3 ductus diverticulum or ductus arteriosus aneurysm, since there are no other large scale studies or definitions available in adult literature.
Patent ductus arteriosus have been historically closed using Amplatz occluder devices and surgical Dacron grafts [1]. Since DAA is extremely infrequent in the adult population there are no clear guidelines regarding the necessity of surgical intervention in the asymptomatic patients. However, given the seldom presentation of aneurysmal rupture and the low operative mortality of 7% in the adult population [7]; Lajos et al recommend surgical resection of the aneurysm. The surgical repair of DAA includes cardiopulmonary bypass and creating a Gott shunt by-pass, and then patching the neck of the aneurysm from inside out [7].
DAA in infants has a different course and significance. DAA is a rare lesion that can be associated with severe complications including thromboembolism, rupture and death. Marfan, Smith-Lemli-Opitz, Trisomy’s 21, 13 and Ehlers-Danlos syndromes are associated with DAA. DAA likely develops in the third trimester likely from an abnormal intimal cushion formation or elastin expression [11].
Lundt et al in 1991 studied the natural history and course of DAA in children. They recommend prompt surgical treatment of all spontaneous DAA in patients older than 2 months of age, and in all patients with postoperative DAA. They also suggest surveillance using echocardiogram after treatment for any recurrence [12].
References:
1. Fernando R, Koranne K, Loyalka P, Kar B, Gregoric I. Patent ductus arteriosus closure using an Amplatzer (™) ventricular septal defect closure device. Exp Clin Cardiol. 2013; 18 (1): e50-4.
2. Ohtsuka S, Kakihana M, Ishikawa T, Noguchi Y, Kuga K, Ishimitsu,et al. Aneurysm of patent ductus arteriosus in an adult case: Findings of cardiac catheterization, angiography, and pathology. Clin Cardiol.1987; 10:537–40.
3. Danza FM, Fusco A, Breda M, Bock E, Lemmo G, and N Colavita. American Journal of Roentgenology 1984 143:1, 131-133.
4. Kokotsakis J, Misthos P, Athanassiou T, Skouteli E, Rontogianni D, Lioulias A. Acute Ortner’s Syndrome Arising from Ductus Arteriosus Aneurysm. Stainback RF, ed. Texas Heart Institute Journal. 2008; 35(2):216-217.
5. Satake S, Okada M, Matsuda H, Ohta T, Yamashita C, Ozawa S, et-al. Aneurysm of non fistulous ductus arteriosus in the adult: a case report. Kyobu Geka. 1989; 42 (2): 169-72.
6. Cheng TO. Aneurysm of a nonpatent ductus arteriosus. An unusual cause of mediastinal mass. Dis Chest. 1969; 55 (6): 497-500.
7. Lajos, Thomas, et al. “Stapling as a method of closure of ductus arteriosus aneurysm in the adult”, Cardiovascular and Thoracic surgery, 2005, 4 (1): 12-14.
8. Ferrera PC, Ghaemmaghami PA. Ductus diverticulum interpreted as traumatic aortic injury. Am J Emerg Med. 1997;15 (4): 371-2.
9. Batra P, Bigoni B, Manning J et-al. Pitfalls in the diagnosis of thoracic aortic dissection at CT angiography. Radiographics. 2000;20 (2): 309-20.
10. Goodman PC, Jeffrey RB, Minagi H, Federle MP, Thomas AN. The Angiographic Evaluation of the Ductus Diverticulum. Cardiovasc Intervent Radiol 1982; 5:1-4.
11. Dyamenahalli U, Smallhorn JF, Geva T et-al. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J. Am. Coll. Cardiol. 2000;36 (1): 262-9.
12. Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur J Cardiothorac Surg. 1992;5 (11): 566-70.







