Felipe Kazmirczak, M.D., Chetan Shenoy, M.B.B.S.
Cardiovascular Division, Department of Medicine, University of Minnesota Medical Center, Minneapolis, Minnesota, USA
Clinical Presentation:
A 57-year-old homeless man presented to the emergency room after a syncopal episode. He reported a two-day history of shortness of breath and upper back pain. He was noted to be hypotensive with a blood pressure of 92/69 mmHg. An electrocardiogram showed ST-segment elevations in the inferolateral leads. Emergent coronary angiography revealed diffuse obstructive disease in the left anterior descending coronary artery, occlusion of the distal left circumflex coronary artery and complete total occlusion of the proximal right coronary artery. The left circumflex lesion was felt to be the culprit and it was treated with balloon angioplasty. Echocardiography revealed a left ventricular ejection fraction (LVEF) of 15-20% with a contained rupture and pseudoaneurysm involving the lateral wall. He was recommended coronary artery bypass graft surgery and repair of the left ventricular (LV) pseudoaneurysm, which he refused. He was discharged with medical treatment and presented with NYHA Class III heart failure symptoms 4 months later. He was agreeable to surgery at this time and for surgical planning, he underwent a cardiovascular magnetic resonance (CMR) study.
CMR findings:
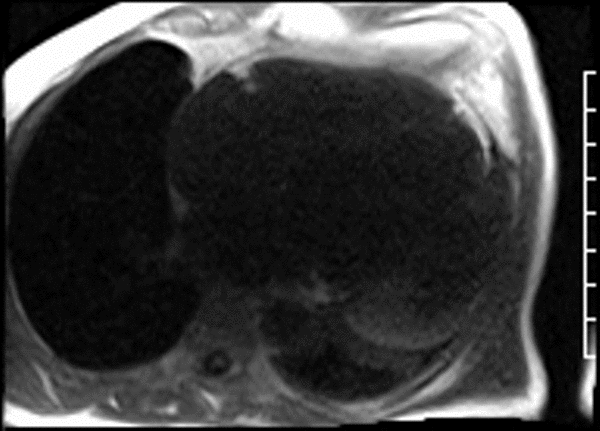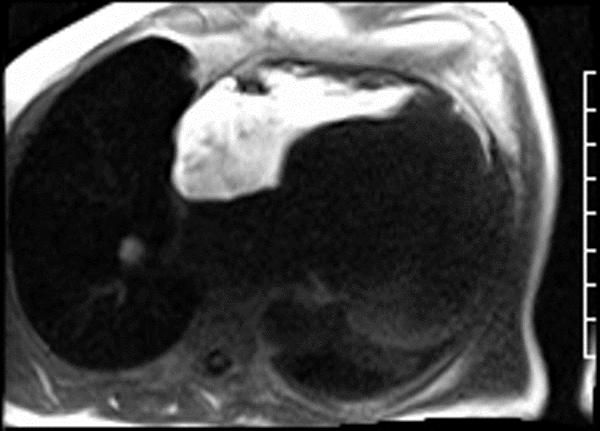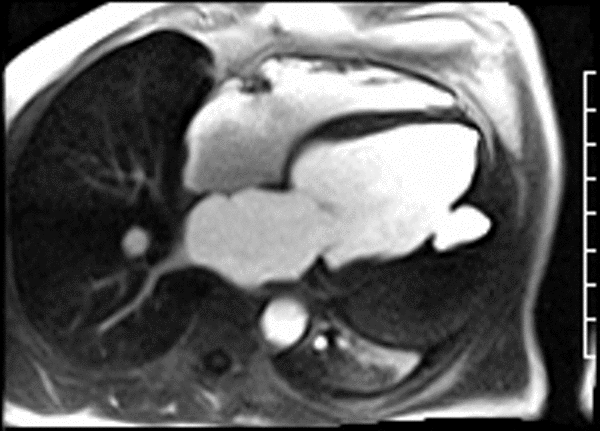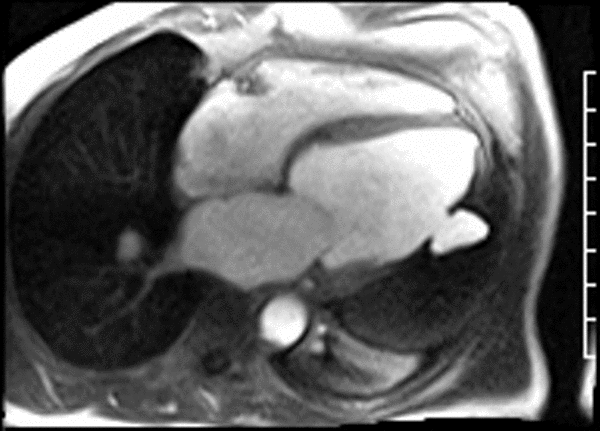
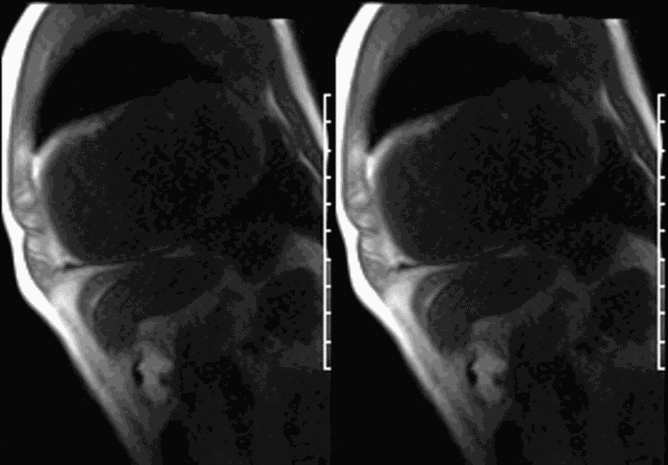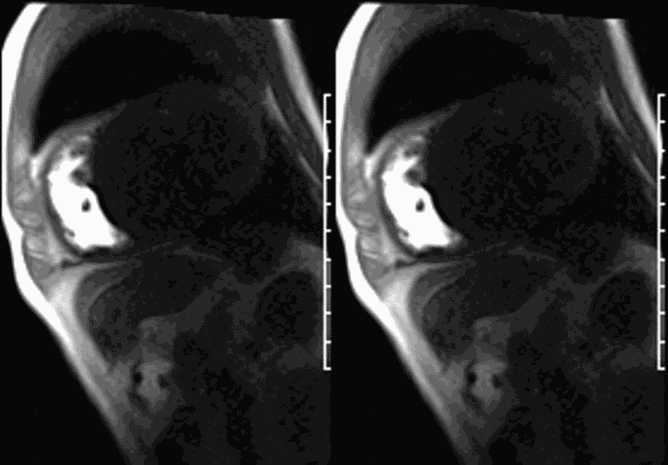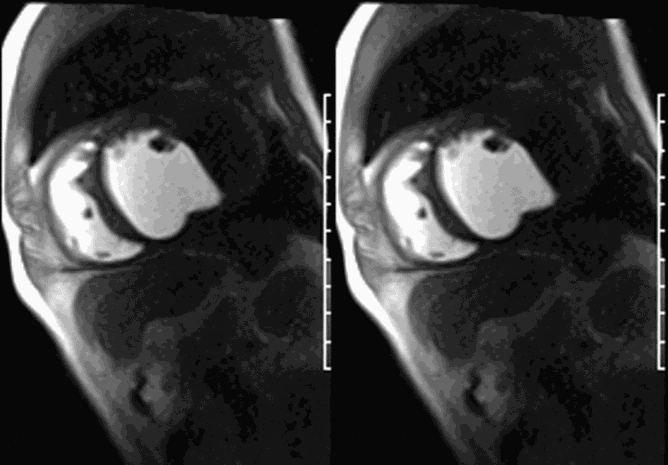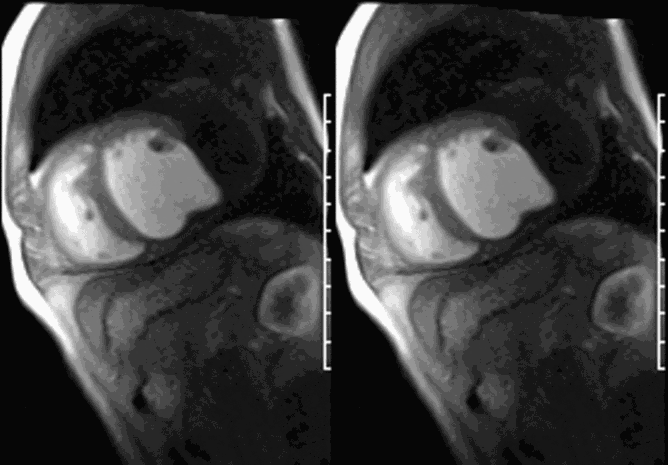
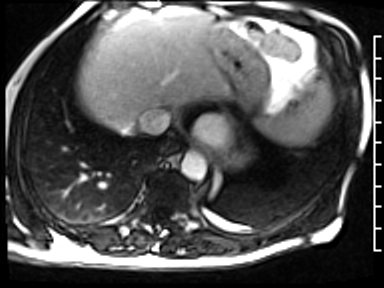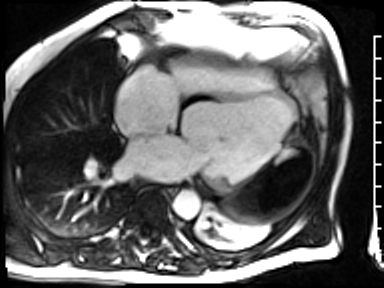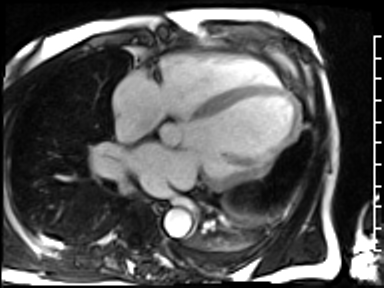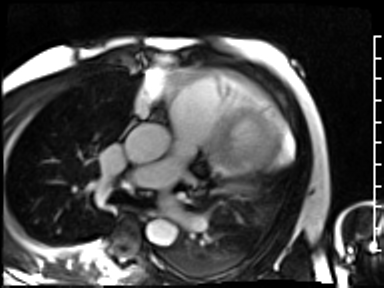
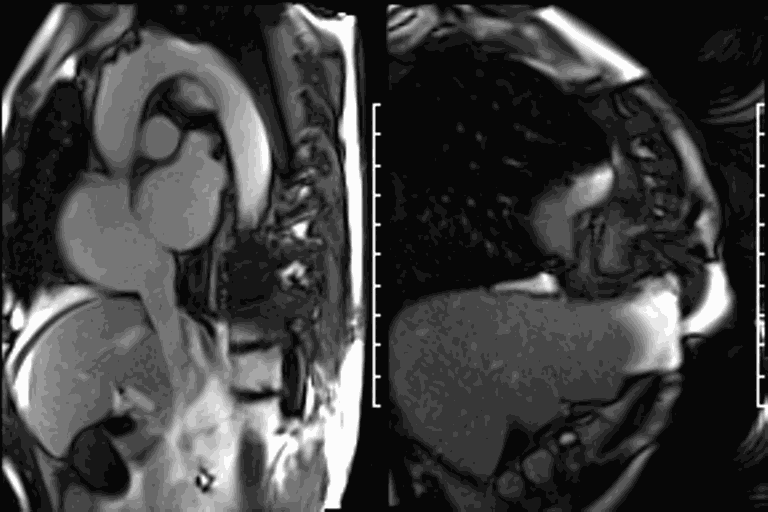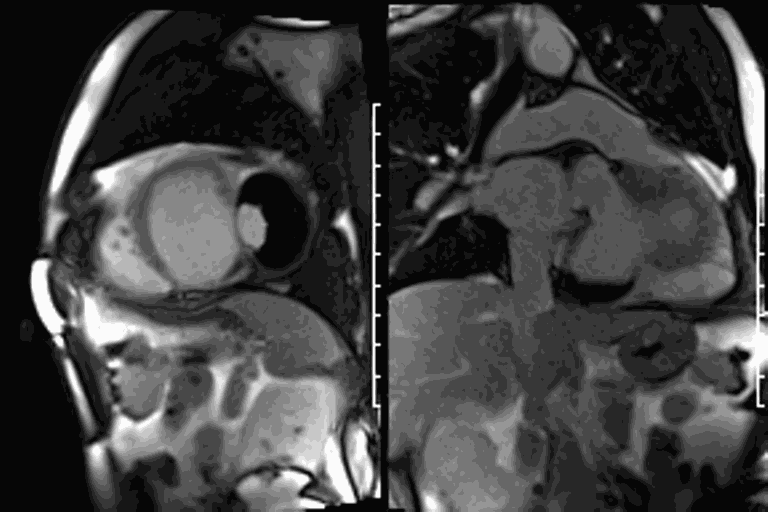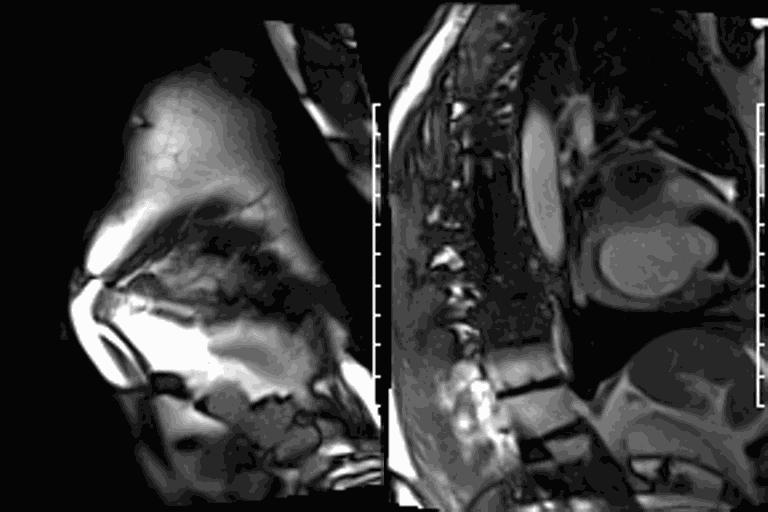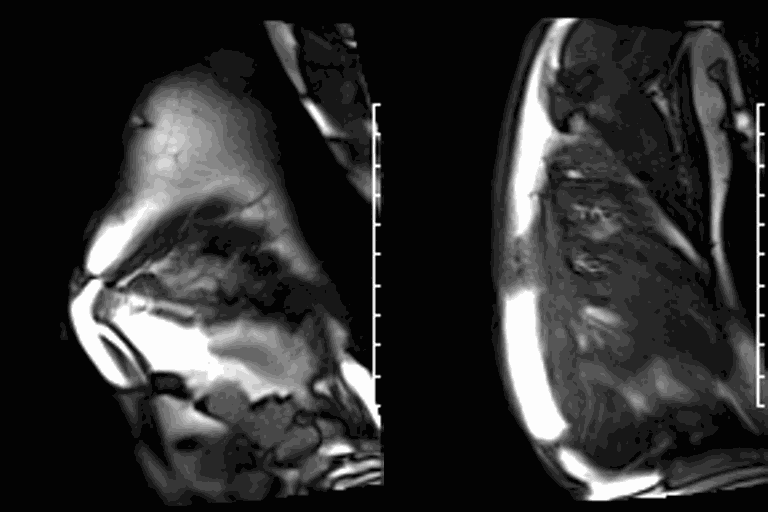
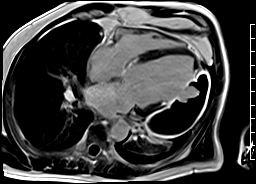
CMR revealed a severely dilated left ventricle cavity with a severely reduced LVEF of 15%. A contained rupture with a giant pseudoaneurysm was seen lateral to the LV. The pseudoaneurysm measured 11.8 cm x 8.7 cm x 4.8 cm in maximal dimensions and 248 mL in planimetric volume. The neck of the pseudoaneurysm measured 2.3 cm x 1.7 cm. Cine CMR (see four-chamber, basal short axis, mid short axis and apical short axis views below) demonstrated flow between the left ventricular cavity and the pseudoaneurysm.
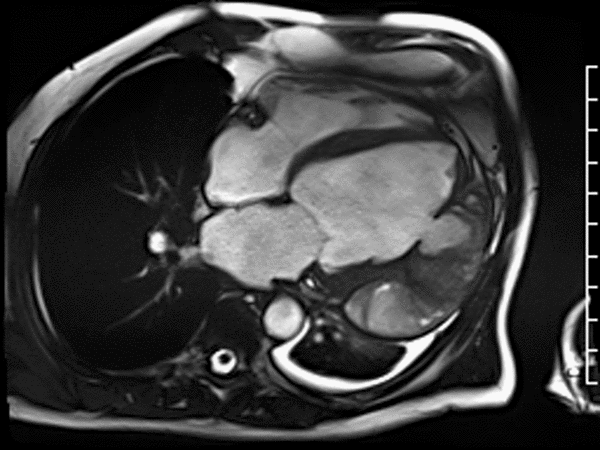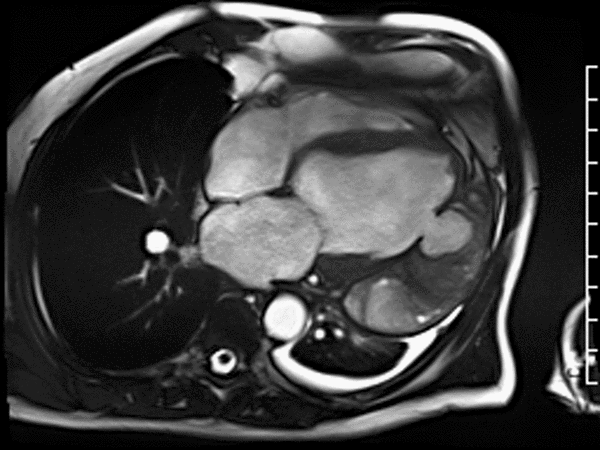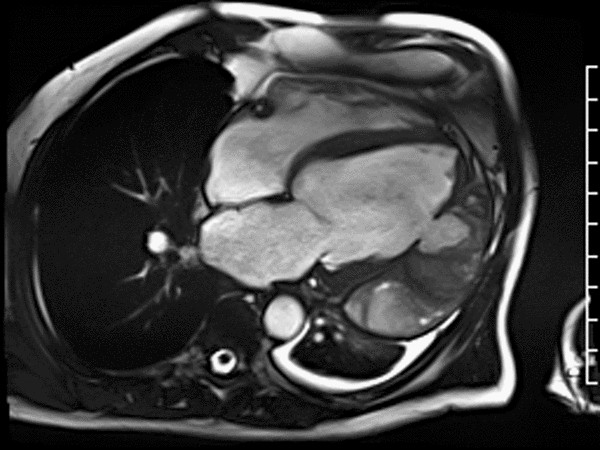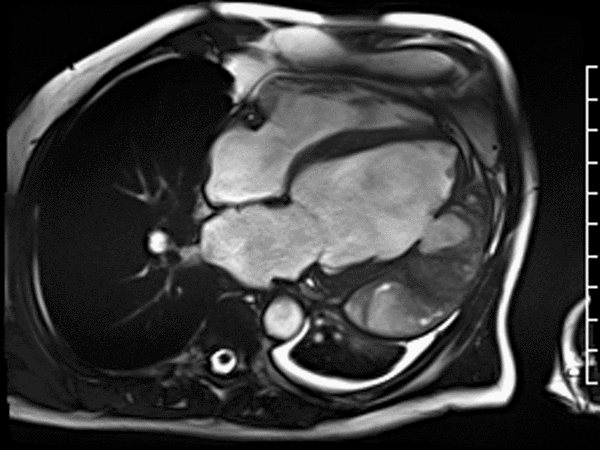
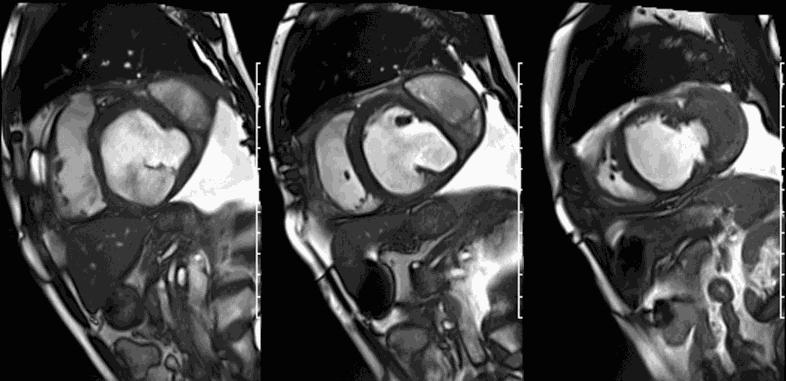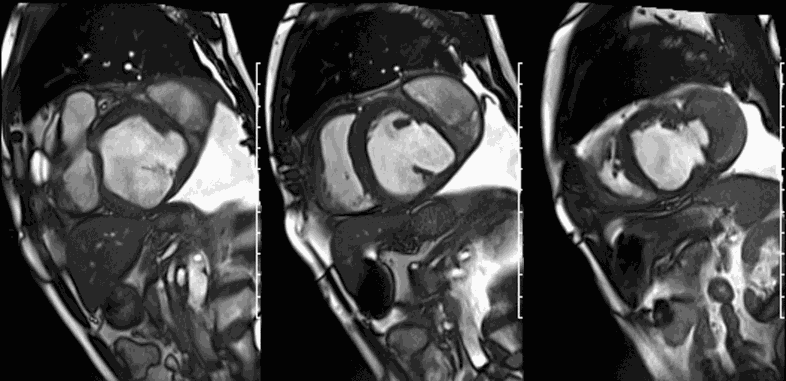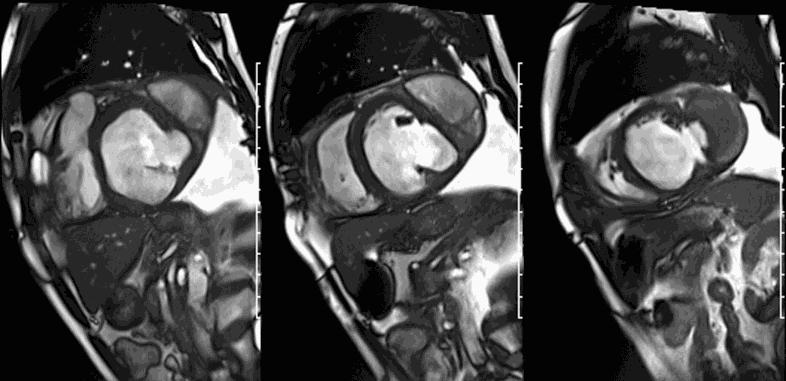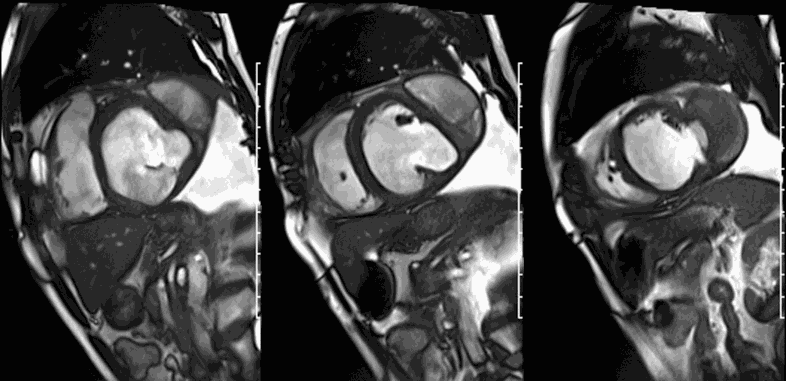
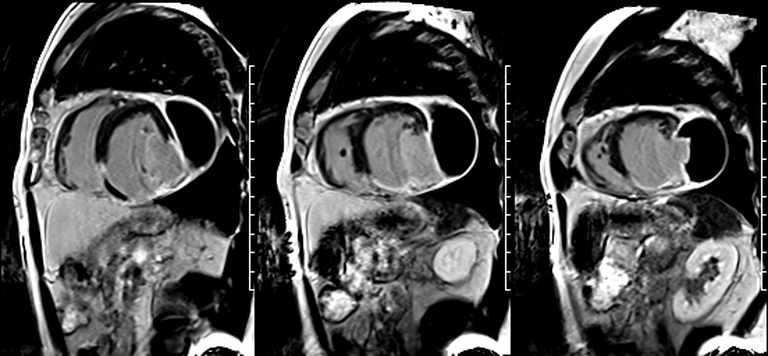
First pass perfusion CMR (see four-chamber, mid short axis and apical short axis views in the first and second rows below) and late gadolinium enhancement CMR with a long TI of 600 ms (long-TI LGE CMR) (see four-chamber, short axis and two-chamber stacks in the third and fourth rows below) revealed a large thrombus burden within the pseudoaneurysm with a planimetric thrombus volume of 225 mL, and a second smaller thrombus adjacent to the basal inferoseptal segment.
LGE CMR [see phase-sensitive inversion recovery (PSIR) four-chamber, basal short axis, mid short axis and apical short axis views below] demonstrated transmural myocardial infarctions in two coronary artery territories – the first involving the inferior and inferoseptal segments, consistent with the right coronary artery territory, and the second involving the lateral segments, consistent with the left circumflex coronary artery territory.
Conclusion:
Five months after his initial presentation, the patient underwent surgical repair of the pseudoaneurysm with a Hemashield patch and coronary artery bypass graft surgery with a left internal mammary artery to the left anterior descending artery. His postoperative course was uneventful and he had improvement in his heart failure symptoms to NYHA Class II. A follow up echocardiogram 5 weeks later showed a LVEF of 20-25% and he had an implantable cardioverter defibrillator implanted for primary prevention of sudden cardiac death. He had no further cardiac events or hospitalizations and died 21 months after his surgery from inoperable invasive squamous cell cancer of the tongue.
Perspective:
Long-TI LGE imaging is currently considered the gold standard for detection of intracardiac thrombus. It is performed using a modified LGE sequence with a fixed inversion time of 600 ms, designed to null avascular tissue such as thrombus. It has been demonstrated to be superior than echocardiography for the detection of small and mural thrombi, which are often adherent to LV aneurysms and pseudoaneurysms (1).
Thrombi can organize over time with gradual replacement by fibrin and collagen (2). This process may involve neovascularization, resulting in vascularity at first, before becoming fibrous and poorly vascular.
Pathologic descriptions of the organization of intracardiac thrombi are rare in the contemporary literature. LGE imaging characteristics of organized thrombus have not been described. One prior study reported that organized thrombus can show inhomogeneous LGE (3). However, this study included only five patients with LV thrombus and imaging was performed with a pulse sequence with poor temporal and spatial resolution, and limited T1-weighting compared with current LGE imaging.
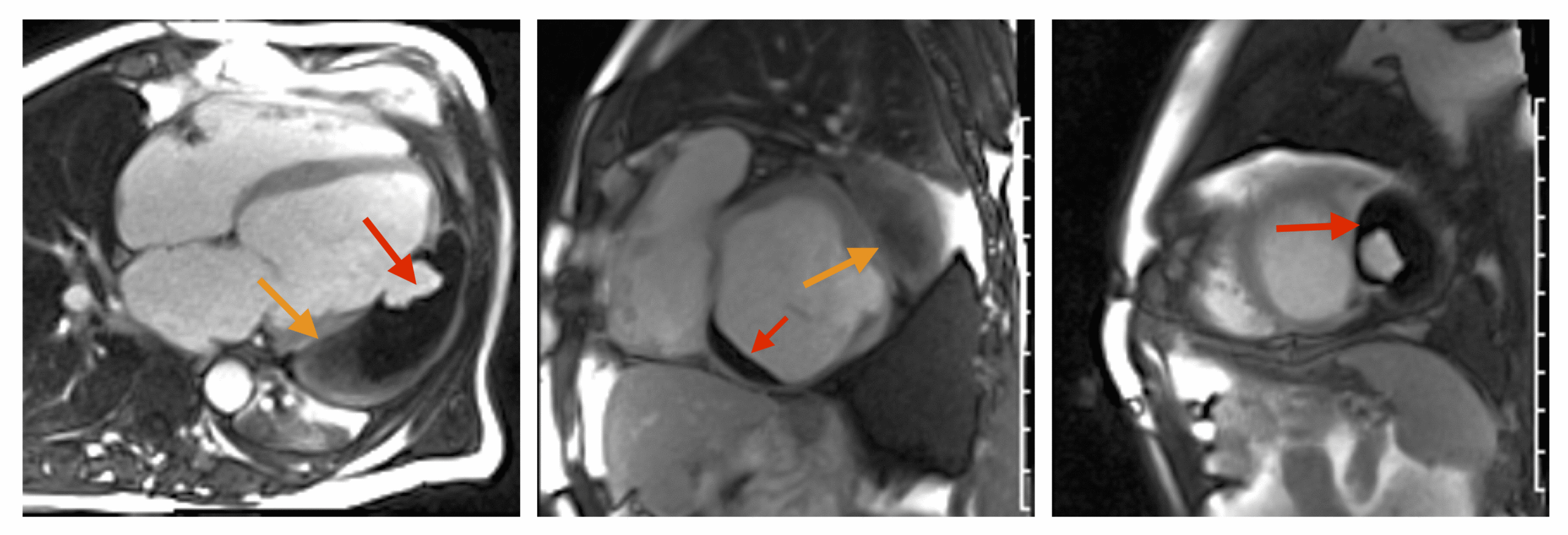
Our case of a giant LV pseudoaneurysm with a large thrombus burden offers rare insights into the natural history of intracardiac thrombus on long-TI LGE imaging. The relatively increased signal in the “oldest” portion farthest from the neck, consistent with contrast uptake, is suggestive of relative organization of this portion of the thrombus. The “signal gradient” between the “freshest” and the “oldest” portions of the thrombus demonstrates the gradual transition between unorganized and organized thrombus.
Click here to view all CMR images for the case on CloudCMR
References:





