Sachin K. Aggarwal (1), Daniel E. Clark, MD, MPH (2), Sean G. Hughes, MD (2)
1. Vanderbilt University School of Medicine, Nashville, TN, USA
2. Vanderbilt University Medical Center, Division of Cardiovascular Medicine, Nashville, TN, USA
Clinical History
A 46-year-old female with a history of asthma and Tetralogy of Fallot was evaluated for progressive dyspnea, chest pain, and palpitations. She underwent transannular patch repair at age 8. Ten years prior to presentation, due to severe pulmonic regurgitation, RV dilation (RVEDVi 180 mL/m2), and declining RV systolic function (RVEF 37%), she underwent bioprosthetic pulmonary valve replacement (PVR; 29 mm Mosaic), aneurysmectomy, and RV outflow tract (RVOT) revision with placement of a Gore-Tex patch to enlarge the RVOT diameter.
On current presentation, the cardiovascular exam revealed a single S1, widely split S2 varying with respiration due to right bundle branch block, 1/6 systolic ejection murmur and early-mid diastolic murmur best heard at the left sternal border. Holter monitor identified sinus rhythm with frequent premature atrial contractions, occasional ectopic atrial rhythm, occasional premature ventricular contractions, and one 10-beat run of asymptomatic ventricular tachycardia. Transthoracic echocardiogram (TTE) compared to prior TTE showed a 5-10% decline in LV ejection fraction due to global mild hypokinesis, mild-moderate aortic valve insufficiency, and mildly low RV ejection fraction due to akinesis of the RVOT. Lasix was started, yielding resolution of paroxysmal nocturnal dyspnea but dyspnea upon walking 25-50 meters on flat ground persisted. She was referred for right and left heart catheterization, which demonstrated mildly elevated right heart filling pressures (RV 75/20; RA mean 12), moderate pulmonary hypertension (59/33, mean 41), and severely elevated left-sided pressures (LV end diastolic pressure (LVEDP) 41 mmHg) with systemic hypertension (aortic pressure 161/85 mmHg). Selective coronary angiography revealed no obstructive coronary artery disease. Intravenous diuretics and afterload reduction were initiated. Cardiac magnetic resonance (CMR) was obtained for a definitive assessment of biventricular volumes, function, and tissue characterization.
CMR Findings
CMR (Images 1-6) revealed a mildly dilated left ventricle (LV end diastolic volume index (EDVI) 115 mL/m2) with moderately depressed systolic function due to global hypokinesis (LVEF 35%), mildly dilated right ventricle (RVEDVi 108 mL/m2) with severely depressed systolic function due to global hypokinesis and infundibular akinesis (RVEF 25%), moderate-severe aortic regurgitation (22 mL, 40% regurgitant fraction, holodiastolic flow reversal in the descending thoracic aorta), moderate tricuspid regurgitation (30% regurgitant fraction), and well-seated 29 mm Mosaic bioprosthetic pulmonary valve replacement (PVR) with mild stenosis (peak velocity estimated 2.8 m/s) without significant regurgitation. A 15 x 53 mm, sessile, laminated thrombus without a mobile component was identified along the akinetic right ventricular outflow tract at the site of the Gore-Tex RVOT patch.
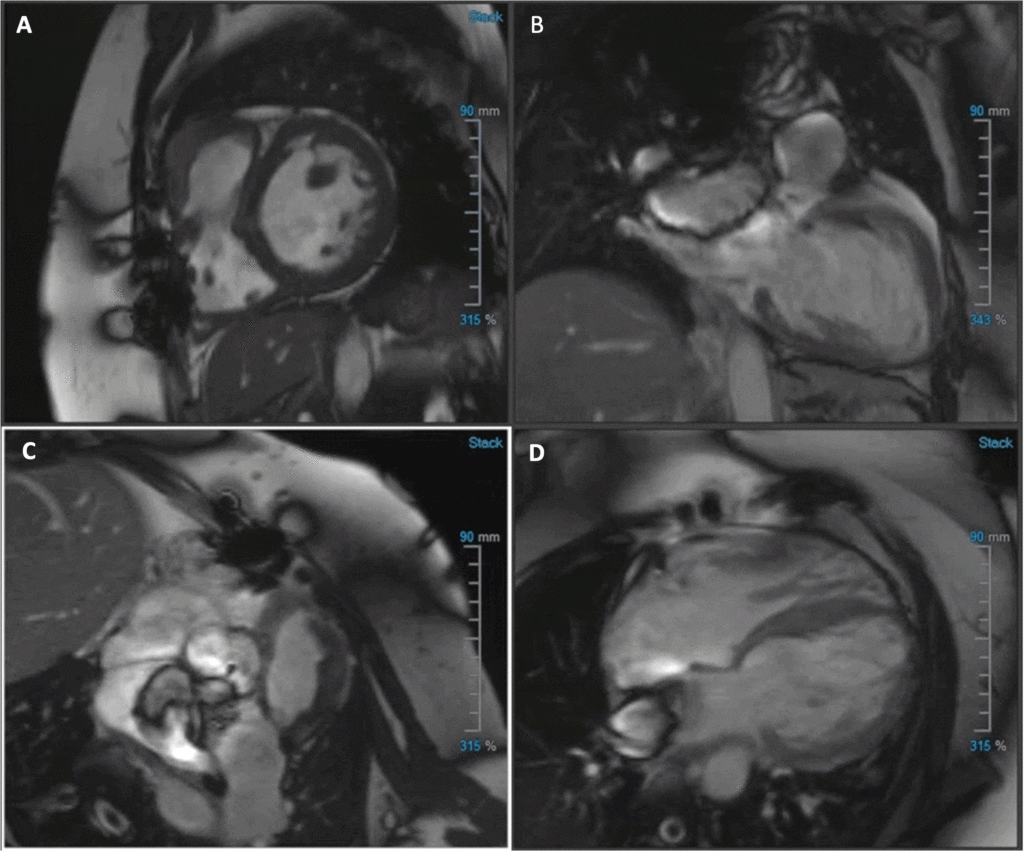
Image 1: (A) Short-axis (SAX) balanced steady state free precession (bSSFP), (B) 2-chamber bSSFP, (C) RVOT bSSFP, (D) 4-chamber bSSFP cine
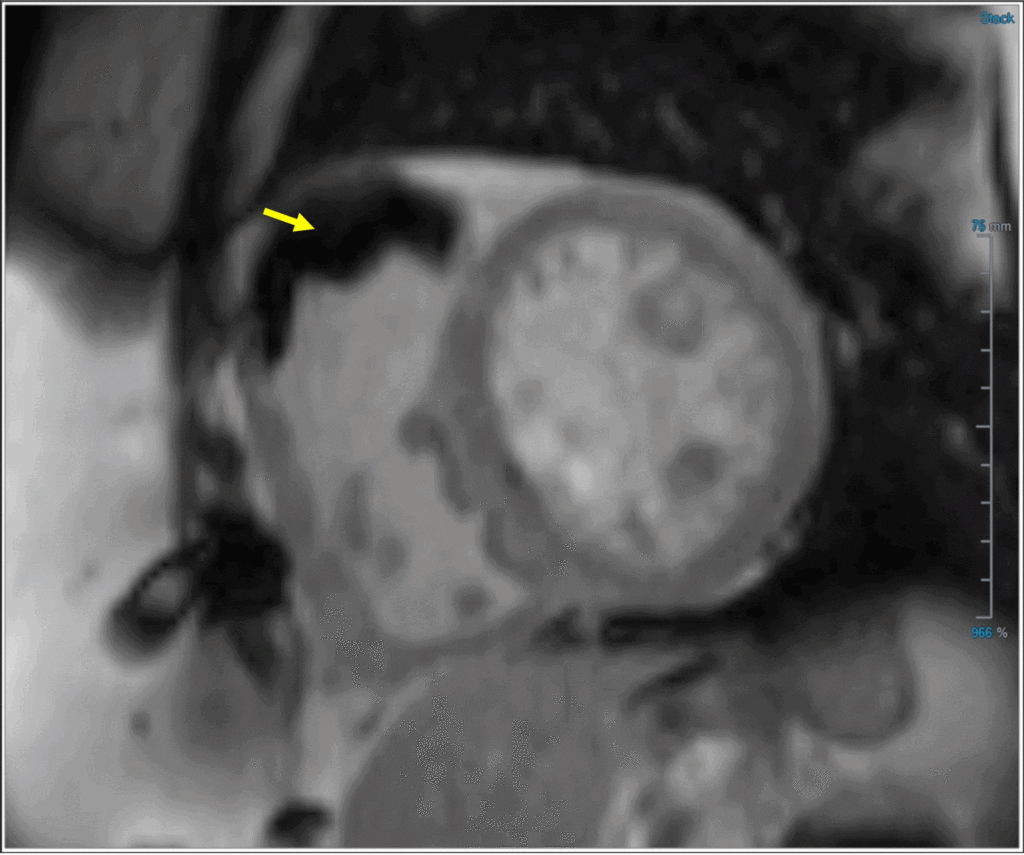
Image 2: Post-contrast inversion recovery image with prolonged TI (600 ms) revealing laminated thrombus in the RVOT (yellow arrow)
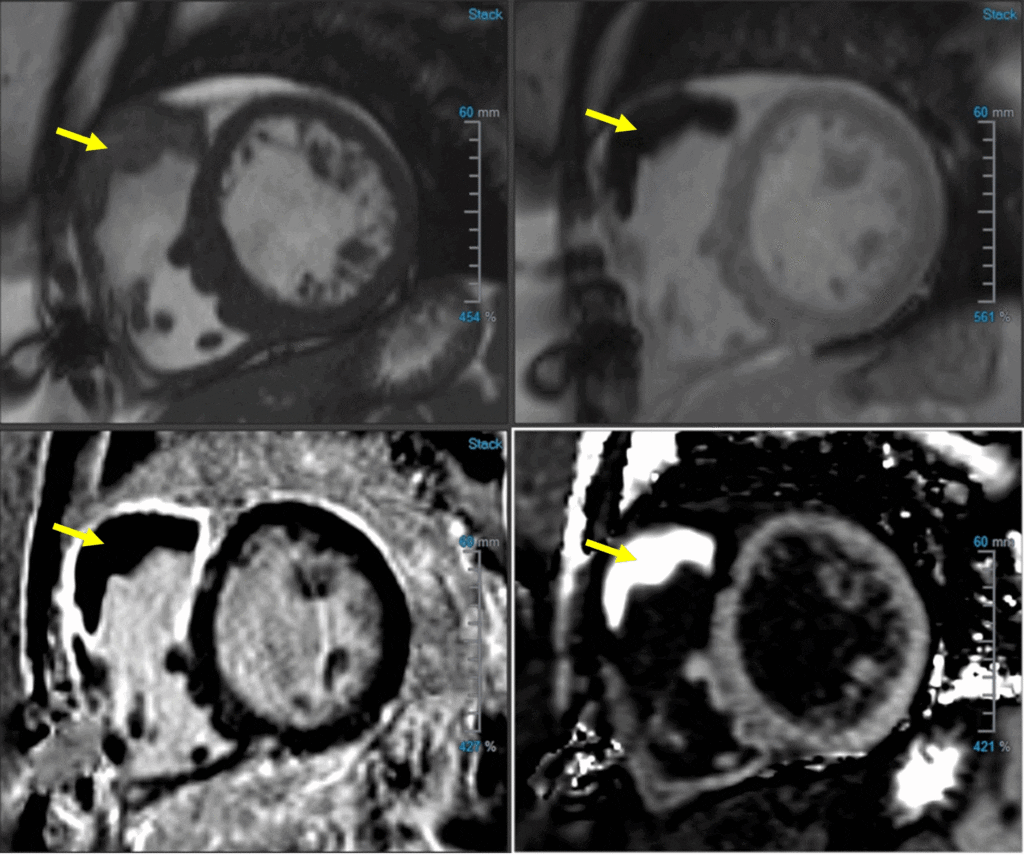
Image 3: RVOT thrombus (yellow arrow) in (A) SAX bSSFP, (B) TI 600, (C) PSIR LGE, and (D) ECV map

Image 4: ECV map of RVOT thrombus (ECV of RVOT thrombus 7%)
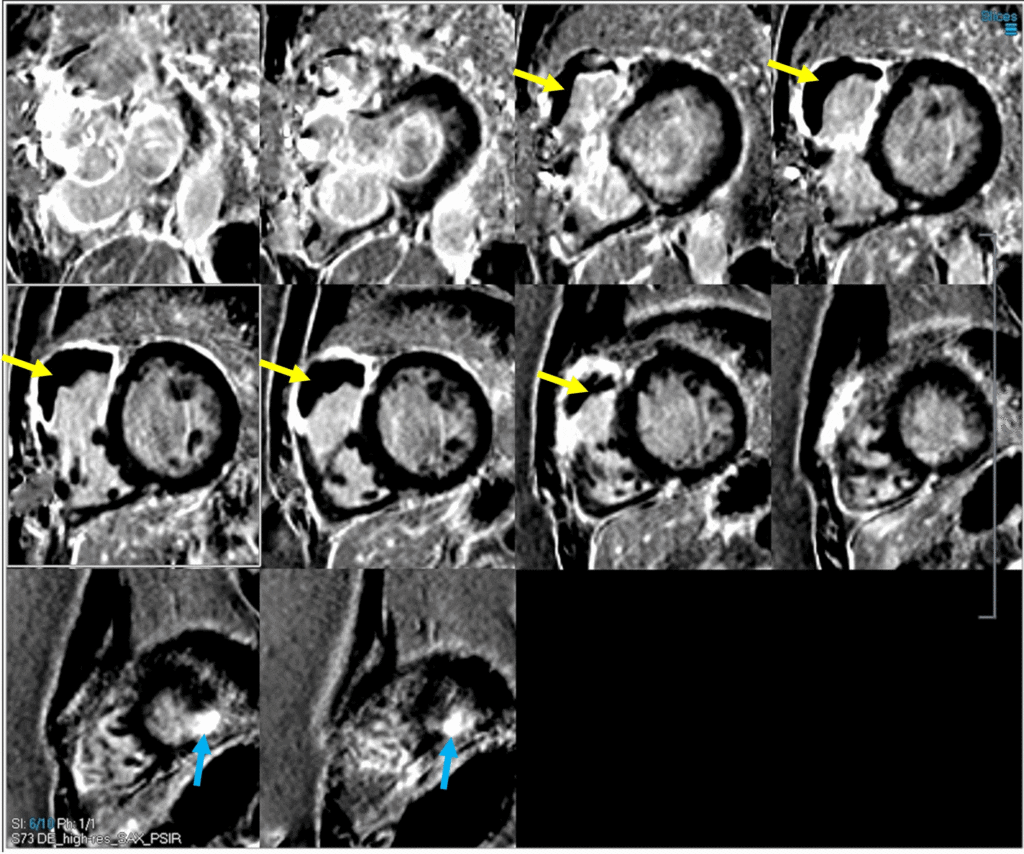
Image 5: PSIR LGE imaging with dark blood technique showing significant basal-mid RVOT, anterosuperior free wall, and RV endocardial anteroseptal (VSD patch site) LGE with a large, laminated RVOT thrombus (yellow arrows). Additionally, there is punctate apical inferolateral transmural LGE from prior left ventricular vent site (blue arrows).

Image 6: SAX CMR image showing RVOT thrombus interposed between transannular patch and VSD patch. Dense LGE is seen at the anterior RV free wall, infundibulum, and endocardial septum.
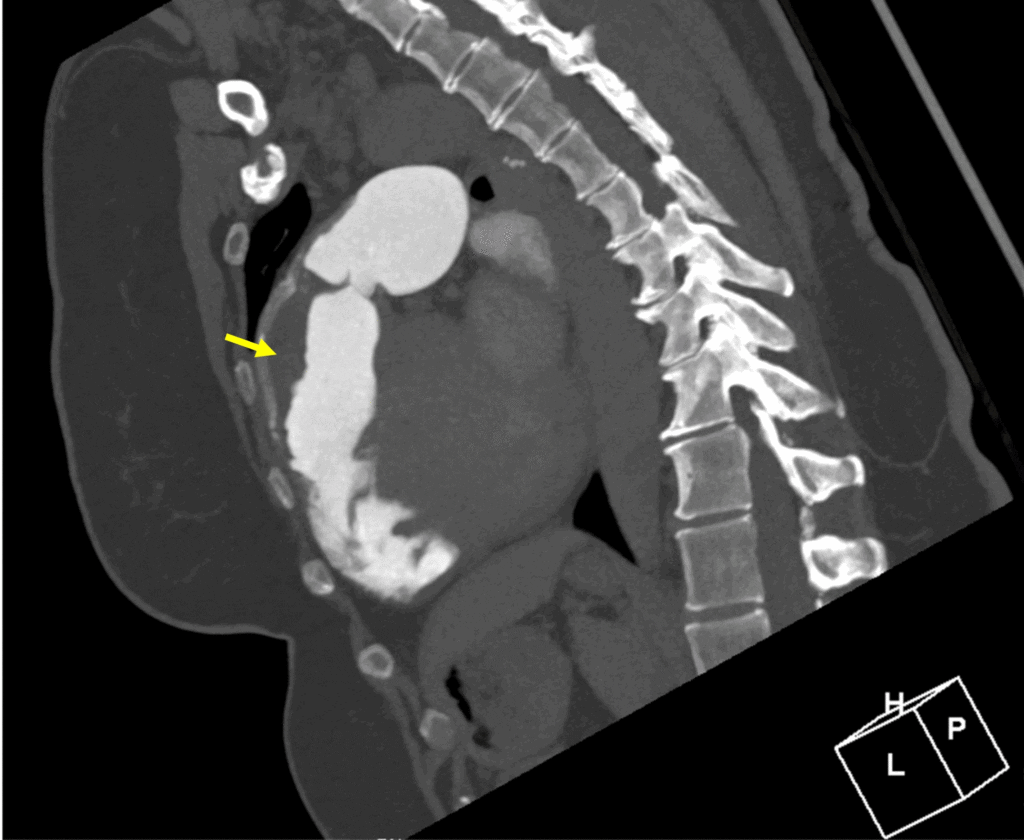
Image 7:
RVOT thrombus by computed tomography angiography (yellow arrow).
Conclusion
Prior to initiating therapeutic anticoagulation for the thrombus identified on CMR, chest CT angiogram was performed and confirmed a laminated thrombus in the RVOT without peripheral embolic changes in either lung and without pseudoaneurysm, perforation, or RVOT disruption (Image 7).
A direct oral anticoagulant and guideline-directed medical therapy for a nonischemic cardiomyopathy were initiated with Apixaban and Entresto.
Three months later, the patient reported improved dyspnea on exertion with no limitation while walking on flat ground. At sixteen-month follow-up, the patient reported resolution of all symptoms. CMR revealed mild LV dilatation, normal LV systolic function (LVEF 55%), normal RV size, low normal RV systolic function (RVEF 44%), mild-moderate aortic regurgitation (regurgitant fraction 16%), bioprosthetic PVR with ongoing mild pulmonic stenosis and no significant pulmonic regurgitation, and resolving, non-mobile, mural laminar thrombus along the RVOT patch measuring 7 x 40 mm (Image 8).
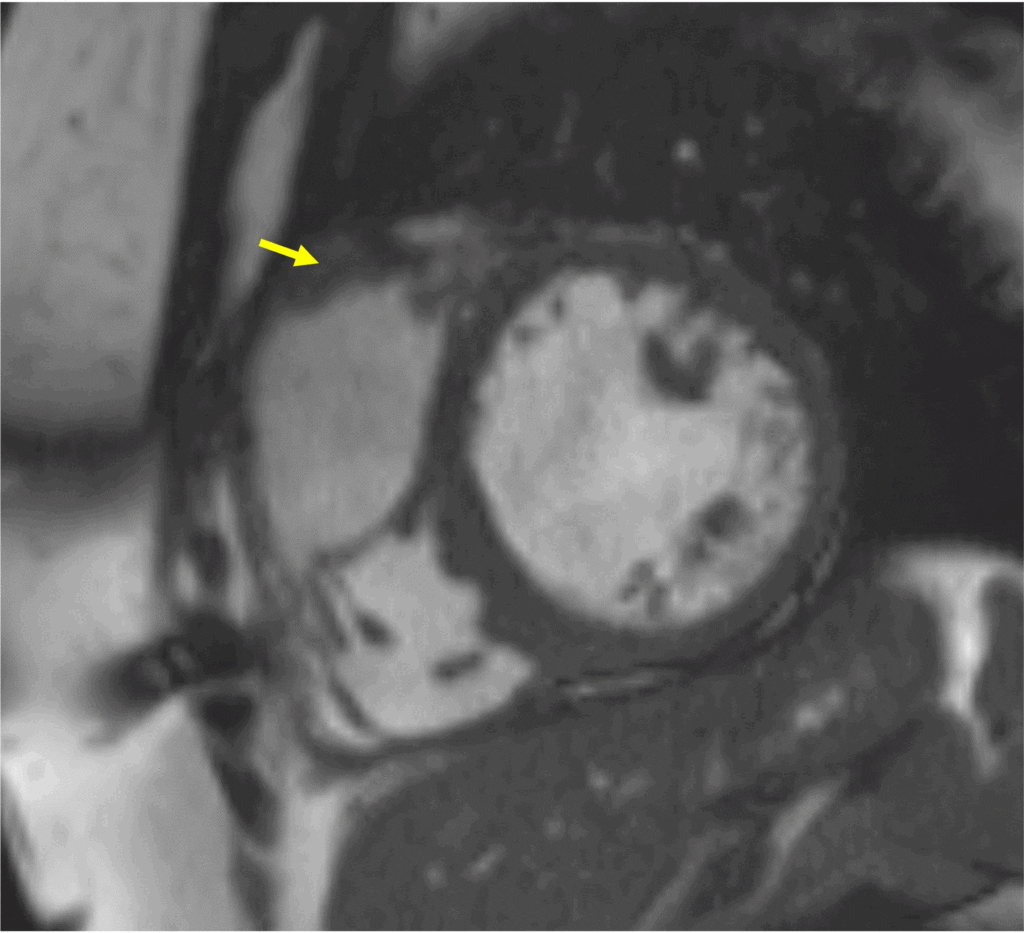
Image 8: Follow-up CMR image showing 7 x 40 mm thrombus (yellow arrow) along the RVOT patch.
Perspective
Isolated, in situ RVOT thrombosis has not previously been reported after RVOT patching in Tetralogy of Fallot, except in the setting of a large RVOT aneurysm (1). The formation of RVOT thrombus in this case likely arose from multiple factors including flow distortions, prosthetic material/abnormal endothelium, and akinesia of the RV free wall. Gore-Tex cardiac patch is a prosthetic material composed of polytetrafluoroethylene that is used as a conduit in various congenital heart disease repairs, including transannular patch for Tetralogy of Fallot repair. Thrombosis associated with Gore-Tex material, although rare, has been reported previously involving a Blalock-Taussig shunt, a central shunt, and Fontan palliation (2, 3, 4).
CMR has been used to investigate flow dynamics in repaired Tetralogy of Fallot, and 4D flow studies have shown a variety of altered flow hemodynamics among these patients (5). During RV systole, increased vortices can be observed in the RVOT because of pulmonary insufficiency countering antegrade systolic flow (6). The interaction of the antegrade systolic jet with the pulmonary regurgitant jet may create variable intracardiac topology that forms shear layers and more turbulent-like flow (7). Abnormal RV direct flow, a measure of blood entering and exiting the ventricle within the analyzed cycle and the main determinant of RV intracavity blood flow kinetic energy, has been shown to be lower in patients with repaired Tetralogy of Fallot in comparison to controls (8). Thus, altered flow dynamics secondary to the nature of Tetralogy of Fallot disease along with RV dysfunction as evidenced by akinesia of the free ventricular wall were likely compounded by the presence of prosthetic material, leading to a milieu predisposing to thrombus formation
With initiation of anticoagulation and guideline-directed medical therapy (GDMT) for non-ischemic cardiomyopathy, there has been sustained, marked improvement in biventricular function. Although counterintuitive at first take, LV systolic dysfunction is more common among adult congenital heart disease patients with biventricular repair and right-sided disease (like Tetralogy of Fallot) than those with left-sided disease (i.e. subaortic stenosis, coarctation of the aorta; 15% vs. 10%, p>0.001) (10). While prior studies have been equivocal about the association of GDMT with improved ventricular systolic function, Egbe et al. found that GDMT improved LVEF and lowered the risk of cardiovascular events among patients with right-sided lesions (including those with Tetralogy of Fallot) (10). Mechanistically, LV recovery may be related to reductions in afterload to both pulmonary and systemic vascular systems (with normotension also helping reduce the degree of aortic insufficiency), improved ventriculo-ventricular interactions, such as improved LV twist, as well as decongestion given this patient’s presentation with hypervolemia and severely elevated left-sided filling pressures. Ventricular interdependence has previously been well-described with RV dysfunction and dilation adversely affecting LV function and geometry, and RV dysfunction secondary to the underlying substrate of repaired Tetralogy of Fallot with akinesia compounded by thrombus in the RVOT likely worsened biventricular dynamics (9). Direct oral anticoagulant use likely aided in resolution of the RV thrombus based on a reduction in size seen on follow-up CMR. Impaired LV twist, not uncommon in Tetralogy of Fallot patients, is suggested by apical late gadolinium enhancement in this patient, and it may also factor into LV dysfunction. CMR was therefore helpful in not only identifying the RVOT thrombus, but also quantifying ventricular performance and guiding medical therapy.
In repaired Tetralogy of Fallot patients, CMR is integral to follow-up imaging for characterization of volumetrics, function, tissue characterization, and flow dynamics while also having utility for evaluating other pathology such as tricuspid regurgitation and aortic insufficiency. CMR further aids in mortality risk prediction and can identify rare complications, such as the RVOT thrombus seen in this case (11).
Click here to view the entire study on CloudCMR
References
1. Peer SM, Bhat PSS, Furtado AD, Chikkatur R. Right ventricular outflow tract aneurysm with thrombus. Interact Cardiovasc Thorac Surg. 2012;14(4):488-490. doi:10.1093/icvts/ivr151
2. Kaur R, Bhurtel D, Bielefeld MR, Morales JM, Durham LA. Cryopreserved Saphenous Vein Compared With PTFE Graft for Use as Modified Blalock-Taussig or Central Shunt in Cyanotic Congenital Heart Disease. World J Pediatr Congenit Heart Surg. 2018;9(5):509-512. doi:10.1177/2150135118776616
3. Chowdhury UK, Airan B, Kothari SS, et al. Specific Issues After Extracardiac Fontan Operation: Ventricular Function, Growth Potential, Arrhythmia, and Thromboembolism. The Annals of Thoracic Surgery. 2005;80(2):665-672. doi: 10.1016/j.athoracsur.2005.02.024
4. Bates O, Semple T, Krupickova S, Bautista-Rodriguez C. Case report of a Gore-Tex TCPC conduit dissection causing severe stenosis. Eur Heart J Case Rep. 2021;5(11):ytab377. doi:10.1093/ehjcr/ytab377
5. Elsayed A, Gilbert K, Scadeng M, Cowan BR, Pushparajah K, Young AA. Four-dimensional flow cardiovascular magnetic resonance in tetralogy of Fallot: a systematic review. J Cardiovasc Magn Reson. 2021 May 20; 23(1):59. doi: 10.1186/s12968-021-00745-0
6. Loke YH, Capuano F, Cleveland V, Mandell JG, Balaras E, Olivieri LJ. Moving beyond size: vorticity and energy loss are correlated with right ventricular dysfunction and exercise intolerance in repaired Tetralogy of Fallot. Journal of Cardiovascular Magnetic Resonance. 2021;23(1):98. doi:10.1186/s12968-021-00789-2
7. Hirtler D, Garcia J, Barker AJ, Geiger J. Assessment of intracardiac flow and vorticity in the right heart of patients after repair of tetralogy of Fallot by flow-sensitive 4D-MRI. Eur Radiol. 2016;26(10):3598-3607. doi:10.1007/s00330-015-4186-1
8. Zhao X, Hu L, Leng S, et al. Ventricular flow analysis and its association with exertional capacity in repaired tetralogy of Fallot: 4D flow cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2022 Jan 3; 24(1):4. doi: 10.1186/s12968-021-00832-2
9. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. Journal of Cardiovascular Magnetic Resonance. 2011;13(1):9. doi:10.1186/1532-429X-13-9
10. Egbe AC, Miranda WR, Pellikka PA, DeSimone CV, Connolly HM. Prevalence and Prognostic Implications of Left Ventricular Systolic Dysfunction in Adults With Congenital Heart Disease. Journal of the American College of Cardiology. 2022;79(14):1356-1365. doi:10.1016/j.jacc.2022.01.040
11. Ghonim S, Gatzoulis MA, Ernst S, et al. Predicting Survival in Repaired Tetralogy of Fallot: A Lesion-Specific and Personalized Approach. JACC Cardiovasc Imaging. 2021 Oct 7:S1936-878X(21)00631-8. doi: 10.1016/j.jcmg.2021.07.026
Case prepared by:
Dr Rebecca Kozor, MBBS PhD
Associate Editor, Cases of SCMR
University of Sydney, Australia





