Nasir Hussain MD1, Raghu R Tadikamalla MD2, Victor Farah MD1
1Allegheny General Hospital, Department of Advanced Cardiac Imaging
2West Penn Hospital
Clinical History:
A 56-year-old African American male with known essential hypertension presented to the emergency department for an evaluation of elevated home blood pressure readings. On review of symptoms, patient reported mild, intermittent, self-limited, non-radiating mid chest tightness that would last for a few minutes and had been ongoing for past few days. There were no aggravating or relieving factors. Rest of review of symptoms were non-contributory. Patient denied any other medical problems and was currently not taking any medications. Patient denied any use of tobacco, alcohol, or any recreational substance. Family history was significant for history of stroke.
His vitals at presentation showed blood pressure of 175/114 mm Hg, heart rate 76 beats per minutes, oxygen saturation 96%, afebrile. Physical examination was within normal limits. His presentation electrocardiogram showed non-specific T wave inversions. His blood work including troponin were within normal limits except LDL was abnormal at 183 mg/dl, and D-dimer was minimally elevated. Computed tomogram of chest with intravenous contrast ruled out any aortic dissection but demonstrated findings that were deemed concerning for LV pseudoaneurysm (figure 1). Patient was admitted as acute coronary syndrome rule out and was treated with antihypertensive medications.
The next morning, his repeat troponin had remained normal. His echocardiogram showed left ventricular (LV) septal abnormality (figure 2 and video 1). Subsequently, patient underwent regadenoson stress test which was negative for any evidence of myocardial ischemia. Patient was discharged to home with plan to perform an outpatient cardiac MR to better assess the LV septal abnormality.
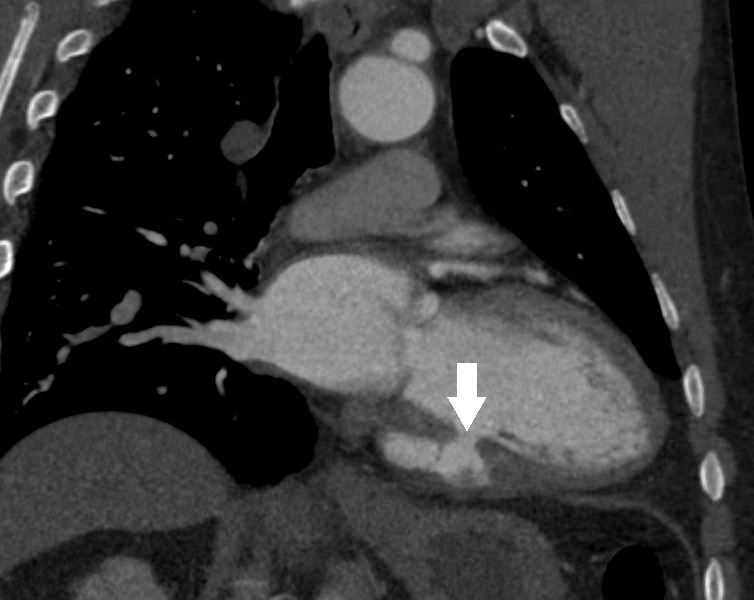
Figure 1. Coronal view of CT demonstrating an LV pseudoaneurysm (white arrow).
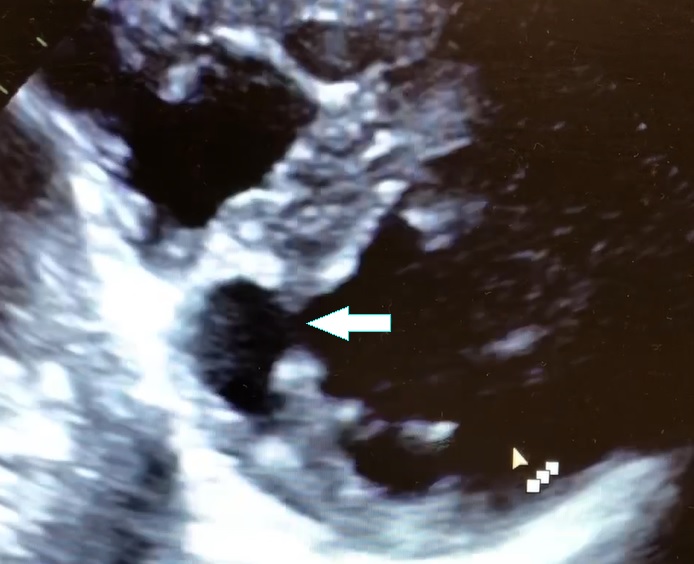
Figure 2. Parasternal short axis view on 2D transthoracic echocardiogram depicting an LV diverticulum inferoseptal.
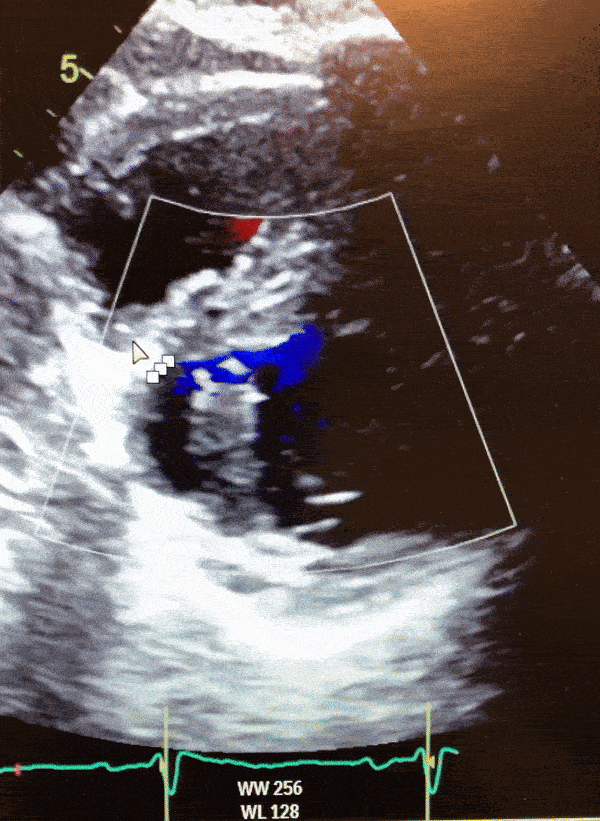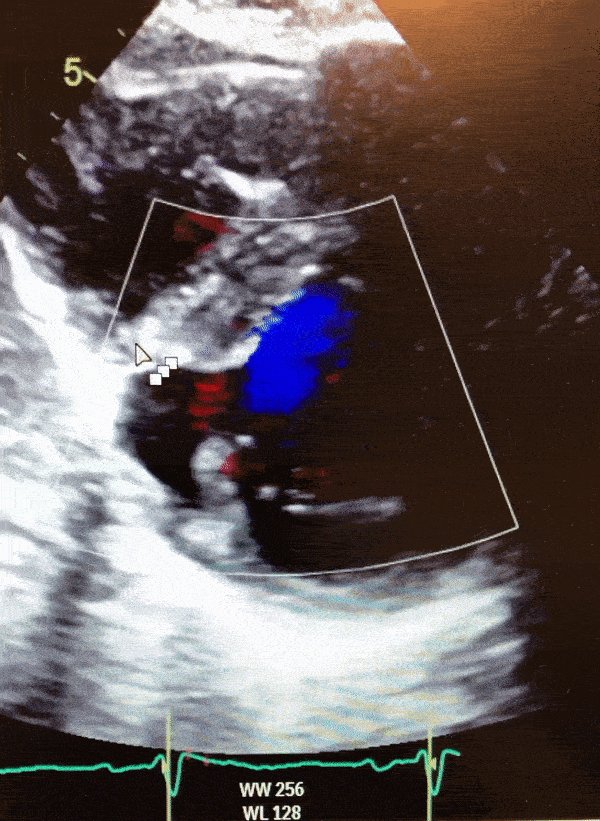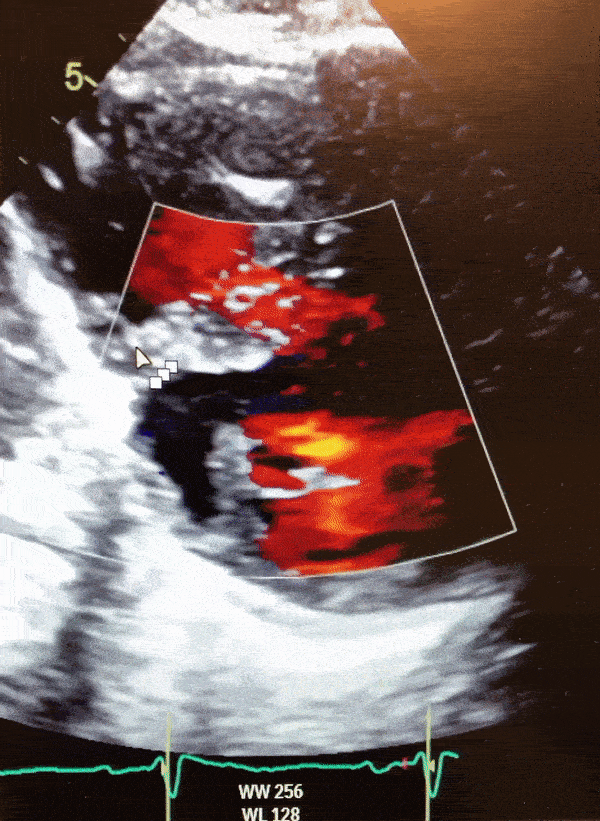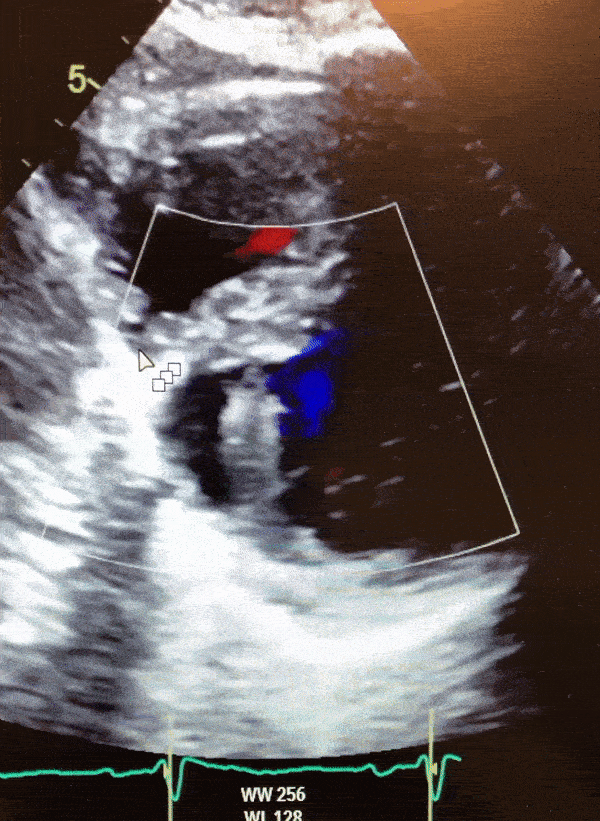
Video 1. Color Doppler parasternal short axis view on 2D transthoracic echocardiogram of the LV diverticulum.
CMR Findings:
Cardiovascular magnetic resonance demonstrated a pouch (21 x 31 mm) at basal to mid inferior and infero-septal segments with a narrow neck measuring 9 mm (figure 3, video 2a, b & 2c).
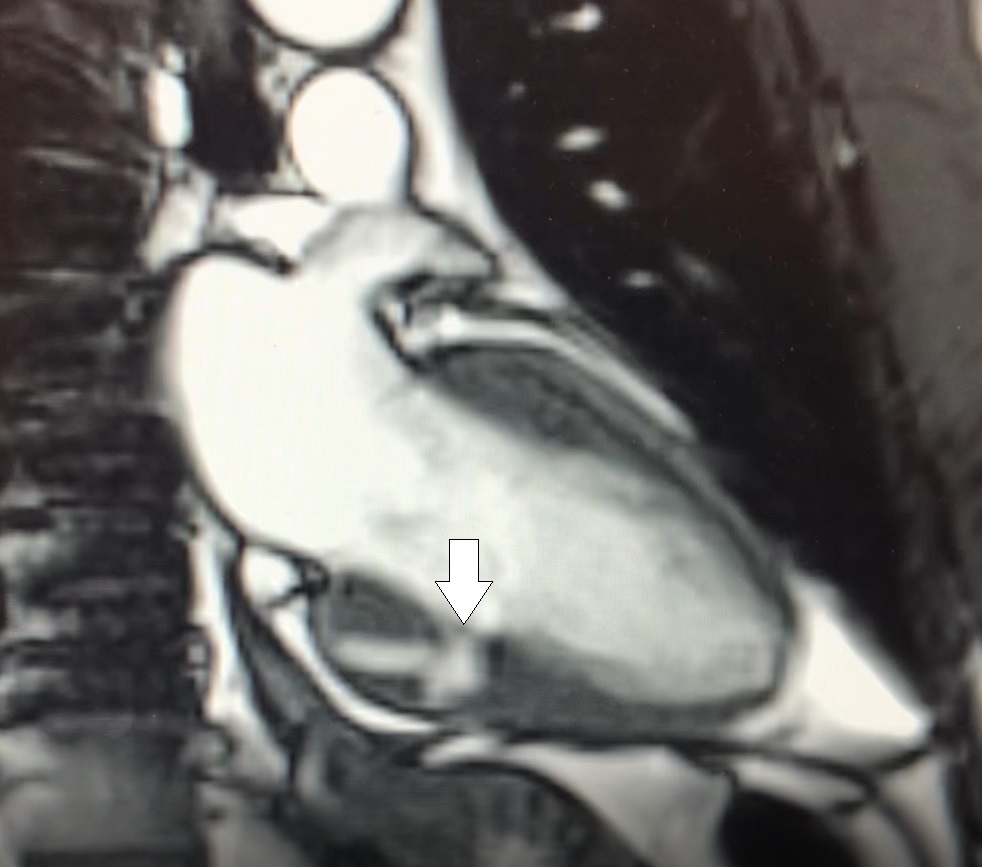
Figure 3. 2-chamber still from SSFP demonstrating pouch with narrow neck involving basal to mid inferior LV segments.

Video 2a. LV pouch on 2-Chamber SSFP.
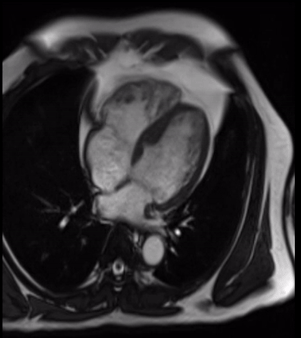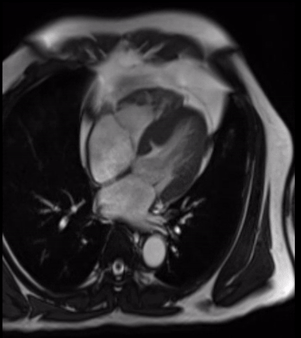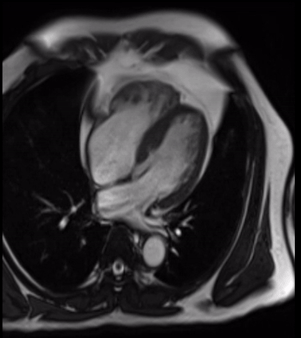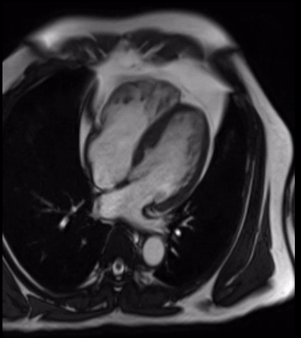
Video 2b. LV pouch on 4-Chamber SSFP.
Video 2c. LV pouch on short axis SSFP.
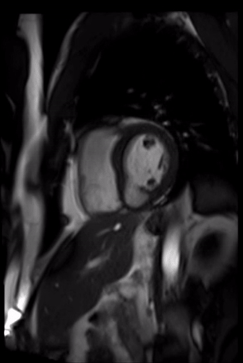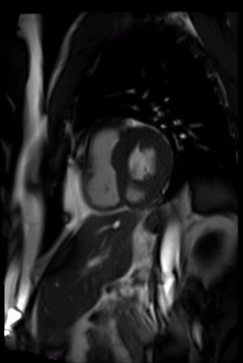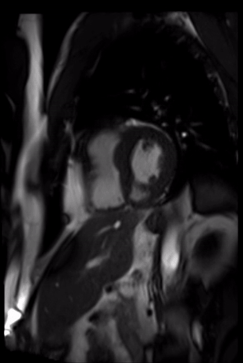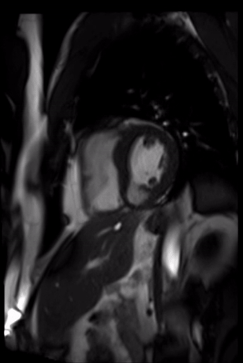
Late gadolinium enhancement acquisitions did not show any clear evidence of infarct, and there was no evidence of any infiltrative or inflammatory process (figure 4).

Figure 4. Late gadolinium enhancement showing normal tissue characteristics on 2-Chamber view.
Conclusion:
Initially, diagnosis of LV pseudoaneurysm was entertained and patient was referred for urgent left heart catheterization and surgical consultation. His left heart catheterization showed no obstructive coronary artery disease. Patient was offered surgical intervention, but declined to proceed with the surgery. Subsequent, cardiac MR at 4-month follow-up showed a stable aspect of the LV pseudoaneurysm (video 3a & 3b).
This case was discussed in multimodality imaging conference and with five different CMR experts. Given, no interval change at 4-month follow-up CMR, and absence of LGE, it was concluded that the abnormality represented congenital LV diverticulum rather than LV pseudoaneurysm. At 7-month follow-up from his initial presentation, patient continues to do well and has not required any (surgical) intervention.
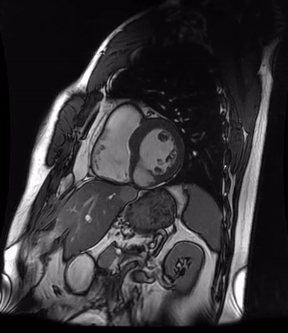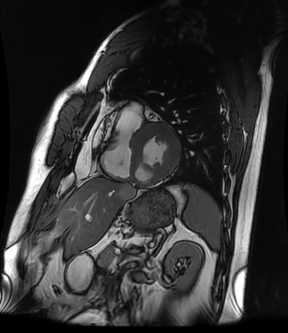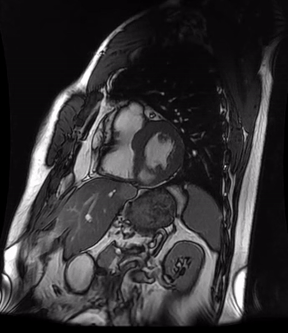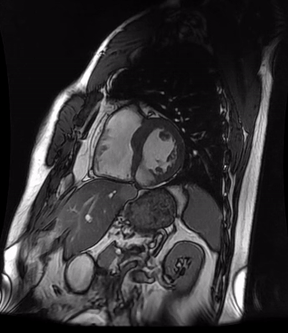
Video 3a. 4-month follow up cardiac MR, LV diverticulum on short axis SSFP sequence.
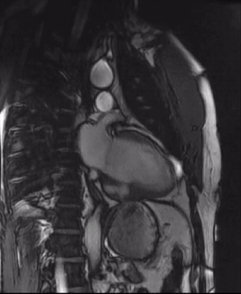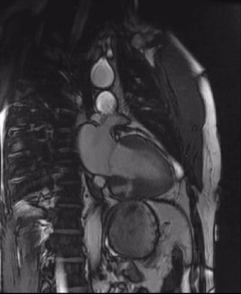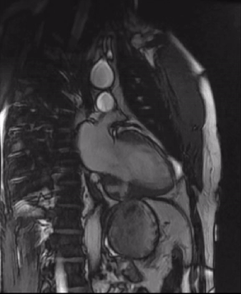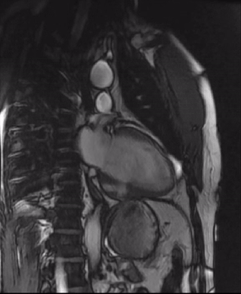
Video 3b: 4-month follow up cardiac MR, LV diverticulum on 2-Chamber SSFP sequence.
LV diverticulum is a rare congenital abnormality that has been reported in 0.4% of cases at autopsy, with its first description dating back to 1816 (1). Congenital LV diverticulum has been classified into 2 different types (1, 2). Muscular type, which contains mostly muscle fibers and usually arises from the LV apex and has synchronous contraction with the LV and has a narrow neck (1, 2). This type may associate with other congenital defects (Cantrell syndrome) (1, 2). The other type is fibrous diverticulum, which mostly consists of fibrous tissue and lacks contractile function (1, 2). Fibrous diverticulum usually involves the inferior basal surface of the LV with a narrow neck (1, 2). Congenital LV diverticulum may be incidentally discovered such as in our case or they may associate with other complications such as arrhythmias, systemic embolism, valvular regurgitation, and spontaneous rupture (1-5). Optimal treatment for congenital LV diverticulum remains elusive with most authors choosing to observe if patient remains asymptomatic (1-5).
It is imperative to make a clear distinction between LV congenital diverticulum, LV aneurysm, LV pseudoaneurysm, and LV crypts, see Table 1. LV pseudoaneurysm usually presents as a complication of myocardial infarction and is defined as a contained myocardial rupture that is contained by adhering tissue, LV pseudoaneurysm depending on clinical circumstances may require a surgical intervention (1-5). Pseudoaneurysm cavity has a narrow neck in sharp contrast to LV aneurysm, which contains all three layers of the LV that balloon out both in systole and diastole and has a wide neck (1-5). LV aneurysm in most cases is medically managed (1-5). Congenital LV diverticulum usually has a narrow neck and may be of muscular or fibrous type, but clinical presentation and LGE findings may differentiate this from LV pseudoaneurysm (1-5). Finally, LV crypts are believed to be congenital in etiology and represent discrete fissures or clefts in compacted myocardium that may completely obliterate during systole (6). Clinical significance of LV crypts remains unclear; however, they are frequently seen in hypertrophic cardiomyopathy and in hypertensive heart disease (6, 7).
In summary, differentiating LV diverticulum from LV pseudoaneurysm, LV aneurysm, and LV crypts may influence clinical decision making and thus medical management.
| Location | Opening | Surrounding Wall | Contractility | Etiology and associations | Delayed gadolinium enhancement | |
| Left ventricular aneurysm | Variable | Wide neck | Endocardium, myocardium, and epicardium-fibrotic walls | Akinetic or dyskinetic with ballooning of involved segments | Usually complication of coronary artery disease. Other causes may include sarcoidosis, hypertrophic cardiomyopathy, iatrogenic, trauma, Chaga’s disease, mucopolysaccharidosis etc. | Present |
| Left ventricular pseudo-aneurysm (Contained rupture) | Variable | Narrow neck | Pericardium | Akinetic | Usually complication of coronary artery disease, other causes may include iatrogenic, trauma, infective endocarditis etc. | Present |
| Left ventricular fibrous diverticulum | Usually basal inferoseptal segment of the left ventricle | Narrow neck | Fibrotic wall composed of reticulin fibers, may have some muscle fiber | Akinetic or dyskinetic | Congenital, and usually does not associate with other congenital anomalies | Present |
| Left ventricular muscular diverticulum | Usually apical segment of the left ventricle | Usually narrow neck but can have wide neck | Endocardium, myocardium, and epicardium | Contracts synchronously with the left ventricle | Congenital, and may associate with midline thoraco-abdominal congenital abnormalities(Cantrell syndrome) | Absent |
| Left ventricular crypts/ clefts/ fissures/ crevices | Usually Inferior or septal wall of the left ventricle | Usually narrow and may completely obliterate during systole | Discrete fissure or cleft in compacted myocardium lined by endocardium | Contracts synchronously with the left ventricle | Congenital, frequently associate with hypertrophic cardiomyopathy and hypertensive heart disease | Absent |
Click here to view the entire study on CloudCMR
References
- Romagnoli A, Ricci A, Morosetti D, Fusco A, Citraro D, Simonetti G. Congenital left ventricular diverticulum: Multimodality imaging evaluation and literature review. J Saudi Heart Assoc 2015; 27: 61-67.
- Sherif HM, Maniar HS, Spadea N, Marge M, Banbury MK. Left ventricular diverticulum mimicking ventricular pseudoaneurysm in an adult. Tex Heart Inst J 2010; 37:584-6.
- Aquaro GD, Bella GD, Strata E, Deiana M, Marchi DD, Pingitore A, Lombardi M. Cardiac magnetic resonance findings in isolated congenital left ventricular diverticuli. Int J Cardiovasc Imaging 2007; 23:43–47.
- Walton-Shirley M, Smith SM, Talley JD. Left ventricular diverticulum: case report and review of the literature. Cathet Cardiovasc Diagn 1992; 26:31–33.
- Archbold RA, Robinson NM, Mills PG. Long-term follow-up of a true contractile left ventricular diverticulum. Am J Cardiol. 1999;83(5):810–813. A11.
Case prepared by:
Pranav Bhagirath, MD PhD
Associate Editor, SCMR Case of the Week
St. Thomas Hospital
London, England







