Daniel E. Clark, MD, MPH1, Benjamin P. Frischhertz, MD1, Frank A. Fish, MD1, Alison L. Bailey, MD2, Michael B. Mikolaj, MD, MPH2, Sean G. Hughes, MD1
1 Vanderbilt University Medical Center, Division of Cardiovascular Medicine, Nashville, TN, USA
2 University of Tennessee College of Medicine Chattanooga/Erlanger Health System, Division of Cardiovascular Medicine, Chattanooga, TN, USA
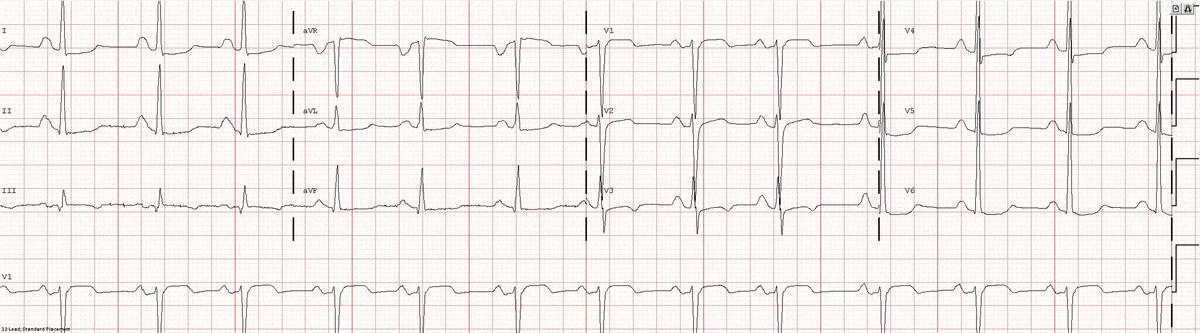
Image 1a: 12-lead ECG revealing sinus rhythm, right atrial abnormality, and diffuse nonspecific ST changes.
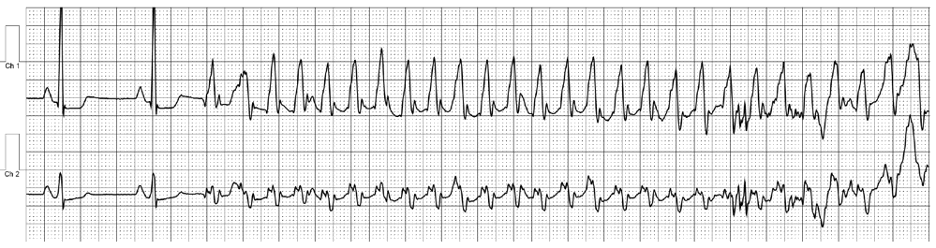
Image 1b: Event monitoring revealing ventricular tachycardia at 240 beats per minute.
Echocardiography revealed preserved left ventricular systolic function despite abnormal septal motion, mild right ventricular dilation, mild right ventricular systolic dysfunction (Image 2), and pulmonic regurgitation without suggestion of residual pulmonic stenosis (peak velocity 0.6 m/s; Image 3). Pulmonic regurgitation was difficult to quantify, and diastolic septal flattening suggested RV volume overload.
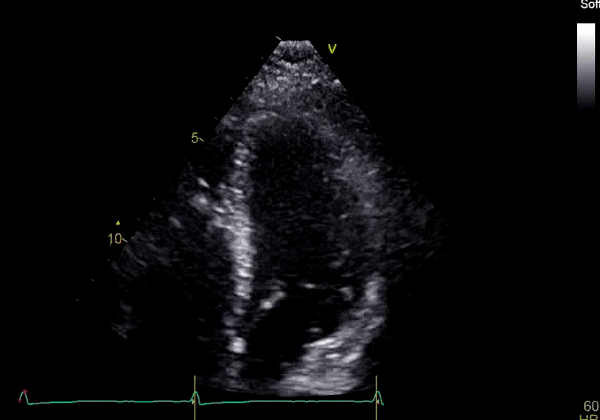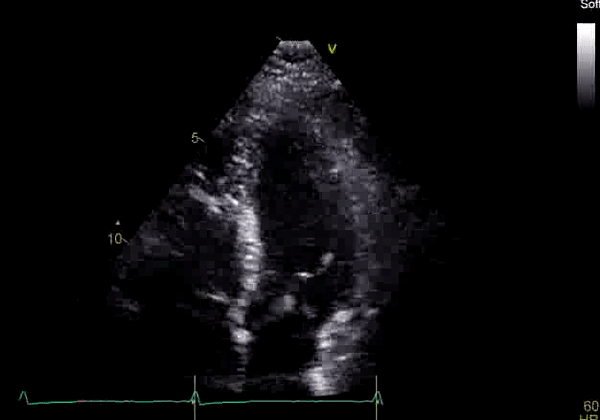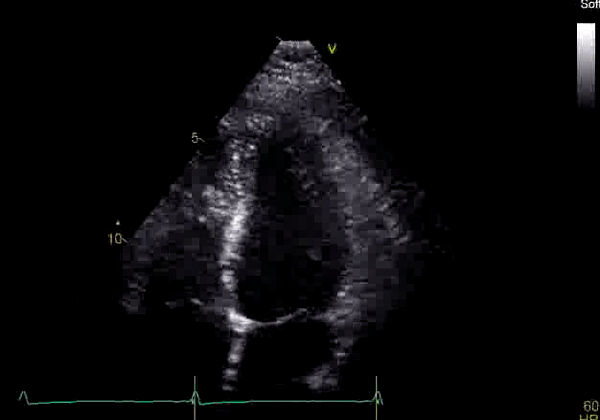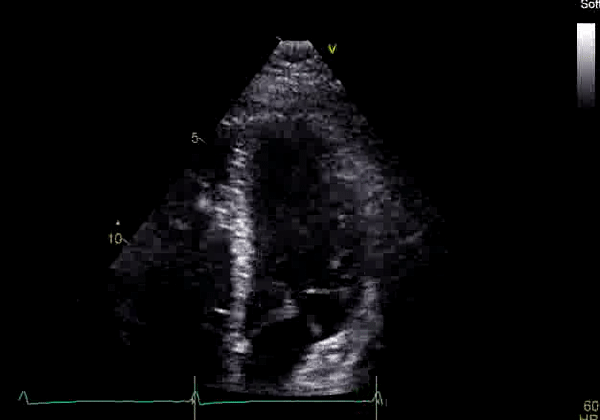
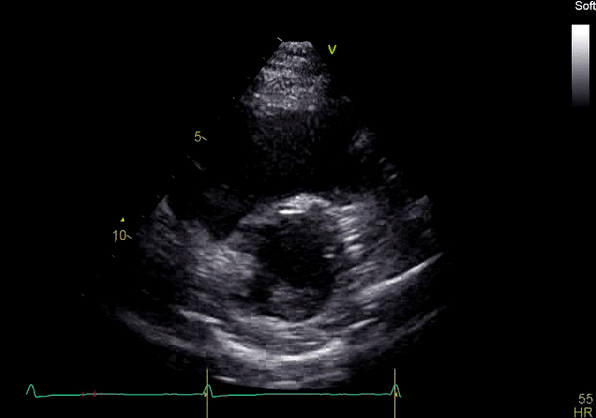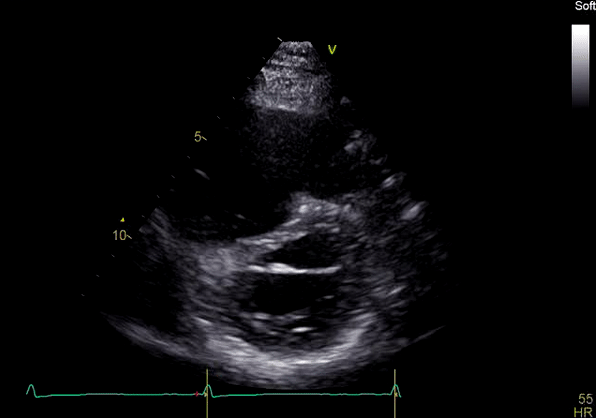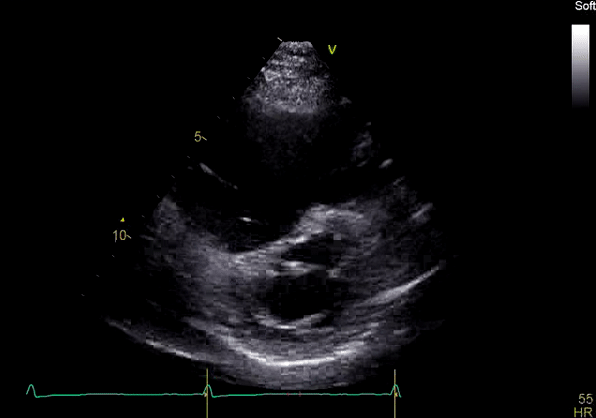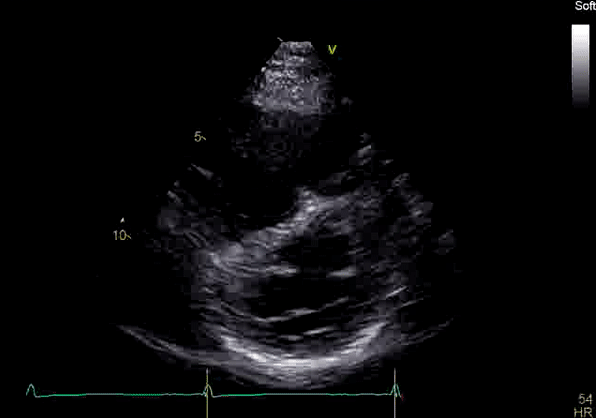
Image 2: Transthoracic echocardiography (TTE) apical four-chamber view (top) and parasternal short-axis view (bottom) revealing RV dilation and diastolic septal shift suggestive of RV volume overload.
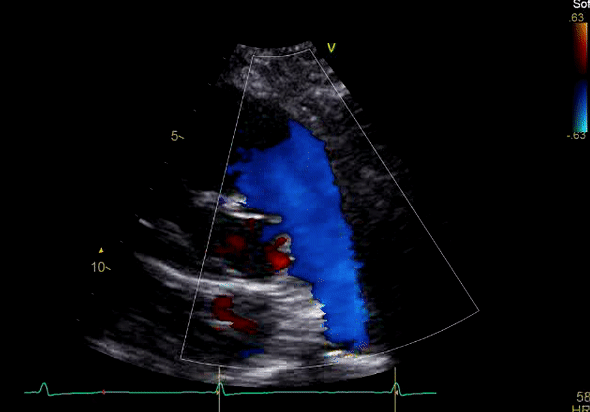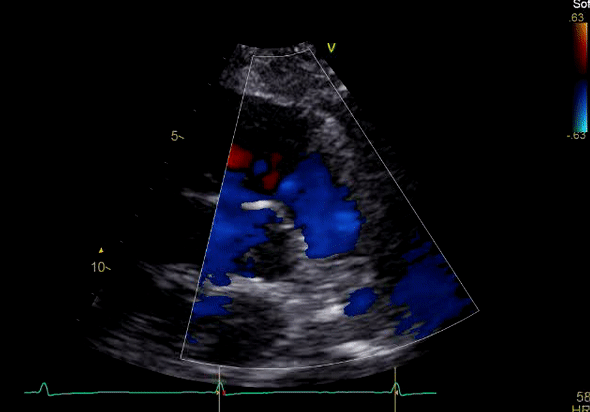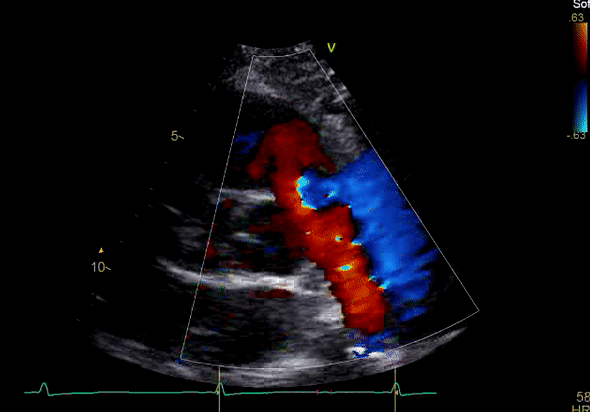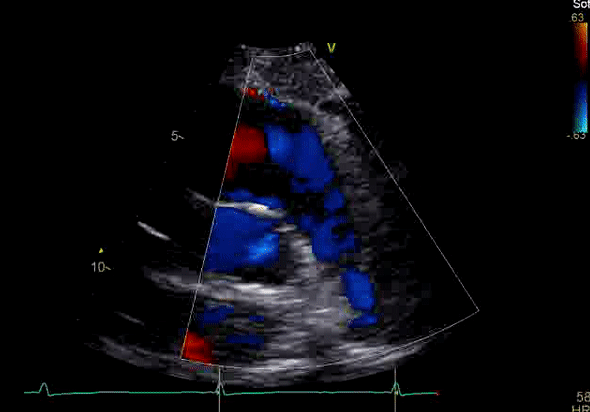
Image 3: TTE, parasternal short axis view, with color Doppler. Pulmonic regurgitation is present but difficult to quantify.
CMR Findings:
Cardiac magnetic resonance (CMR) was requested to comprehensively assess right ventricular (RV) size/function, quantify pulmonic insufficiency, and investigate the etiology of ventricular tachycardia. CMR revealed normal LV size (LVEDVi 63 mL/m2), mild LV systolic dysfunction (LVEF 50%), basal septal thinning and dyskinesis, diastolic septal flattening, normal RV size (RVEDVi 102 mL/m2), moderate RV systolic dysfunction (RVEF 39%), normal LA size (LAVI 26 mL/m2), mild tricuspid regurgitation (14 mL, 18% regurgitant fraction), and moderate pulmonic insufficiency (24 mL, 37% regurgitant fraction) (Images 4a, 4b, 4c).
Scar Imaging: Standard “bright blood” segmented inversion recovery images were not diagnostic, but “dark blood” phase sensitive inversion recovery images (Flow-Independent Dark-blood DeLayed Enhancement: FIDDLE) clearly showed dense right atrial and right ventricular endocardial LGE (Image 5). FIDDLE was first described by Kim and colleagues for use in detection of myocardial infarction.3 The technique relies on selection of an inversion time (T1) with tissue magnetization more than blood, which permits simultaneous myocardial hyper-enhancement and blood-pool suppression. As seen in Image 5, FIDDLE accentuates the contrast between enhancing myocardium and the dark blood pool, and may increase the sensitivity for detection of late gadolinium enhancement of thin-walled structures, such as the atria, right ventricle, and papillary apparatus.
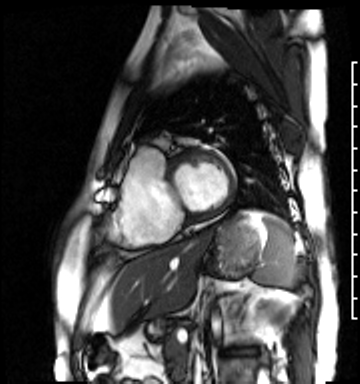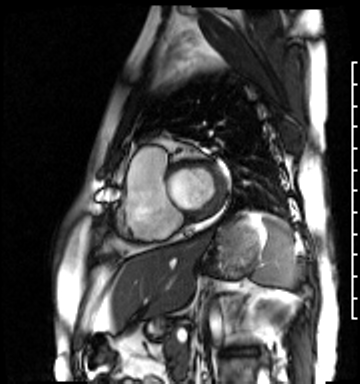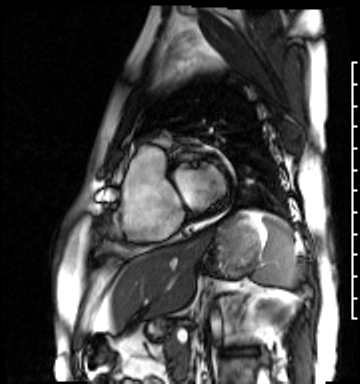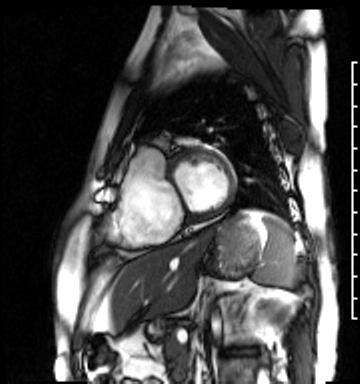
Image 4a: CMR SSFP cine, short axis view, revealing basal septal thinning, systolic septal dyskinesis, and diastolic septal flattening.
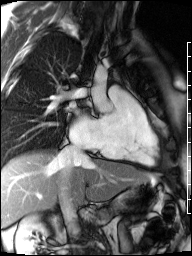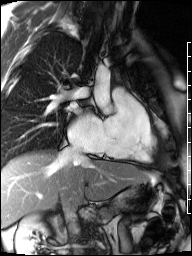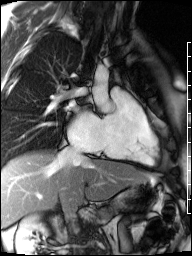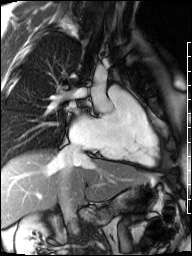
Image 4b: CMR SSFP cine, right ventricular long-axis view, revealing global RV systolic dysfunction and moderate pulmonic regurgitation.
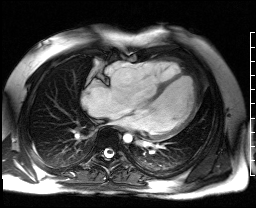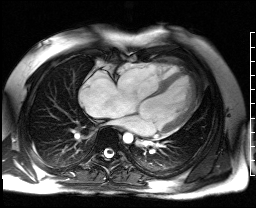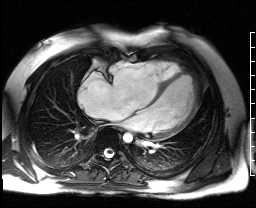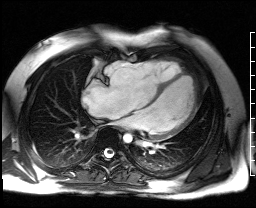
Image 4c: CMR SSFP cine, 4ch view, revealing RV/RA dilation, RV systolic dysfunction, basal septal systolic dyskinesis, and diastolic septal flattening.
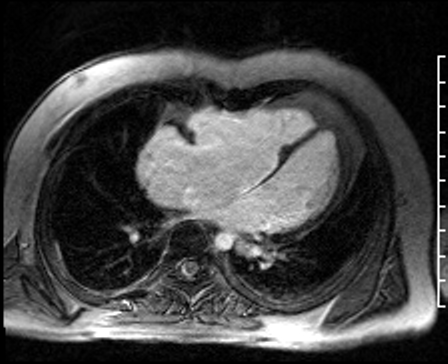
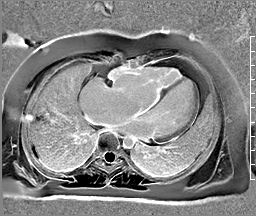
Image 5: CMR scar imaging. Comparison of standard “bright blood” segmented inversion recovery images (top) with “dark blood” technique (FIDDLE, bottom). RV/RA endocardial LGE is readily apparent on dark blood imaging.
A secondary prevention ICD was planned. ICD lead deployment was complicated by unusual difficulty imbedding the fixation coil into the right ventricular (RV) endocardium and abnormally high pacing thresholds. In conjunction with the CMR findings, a diagnosis of right ventricular endocardial fibroelastosis (EFE) secondary to critical, congenital pulmonic stenosis was made.
Perspective:
EFE may be either primary (idiopathic), or secondary to myocardial stress (most commonly aortic stenosis) or inflammatory triggers (1). In this patient, we suspect that EFE developed in utero secondary to critical pulmonic stenosis and elevated right ventricular systolic pressures. Transthoracic echocardiography is not reliable to detect EFE. Two small series of seven children have shown that CMR could detect EFE by LGE imaging (1, 2). This case highlights the improved diagnostic utility of dark blood scar imaging for endocardial LGE assessment as compared to standard bright blood scar imaging (3, 4).
CMR may be a critical modality in the assessment of ventricular tachycardia, especially when associated with right ventricular dilation and/or systolic dysfunction (5, 6). The differential diagnosis of VT in the presence of right ventricular systolic dysfunction is broad and includes arrhythmogenic right ventricular cardiomyopathy, sarcoidosis, infarction, myocarditis, volume loading (congenital shunting, valvular regurgitation), and pressure-loading (pulmonic stenosis, pulmonary hypertension) (7).
In this case, the CMR findings of dense endocardial right atrial and ventricular LGE suggested endocardial fibroelastosis, and this diagnosis was further supported by the abnormal findings during ICD implantation. CMR phase contrast velocity encoded flow imaging is superior to echocardiography in the quantification of pulmonic regurgitation (PR), especially in patients with congenital heart disease ( 5). In this particular patient, CMR revealed that abnormal septal motion was likely primarily due to basal septal thinning and fibrosis, rather than volume overload from severe pulmonic regurgitation. This case highlights the utility of dark blood LGE imaging and flow mapping in the assessment of right ventricular size/function, morphology, and valvular insufficiency in an adult patient with repaired congenital heart disease and ventricular tachycardia.
Click here to review the case on CloudCMR
Acknowledgements: none
Sources of Funding: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007411. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: none
References:
Case prepared by:
Sean G. Hughes, MD
Associate Editor, SCMR Case of the Week
Vanderbilt University Medical Center
Jason N. Johnson, MD
MHS Associate Editor, SCMR Case of the Week
Le Bonheur Children’s Hospital, University of Tennessee Health Science Center







