Héctor A. Carmona-Ruiz+*, Itzel A. Ulloa-Córdoba+, Enrique Santiago-Cerecedo+, Jorge Vázquez-Lamadrid+, Sandra G. Rosales-Uvera+.
+Imagen Cardiovascular, Departamento de Radiología e Imagen. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ).
Av. Vasco de Quiroga No.15, Col. Belisario Domínguez Sección XVI, Del. Tlalpan, CP 14080, Mexico City, Mexico.
* Corresponding author. E-mail: drhacr80@gmail.com
Clinical history
A 24 year old woman with a 3 year history of asthma presented with a disseminated dermatosis manifesting as erythematous, pruritic papules with pain in her metacarpophalangeal and interphalangeal joints. She received unspecified treatment with partial response. Six months later she presented again with erythematous, pruritic papules involving the back, both arms and hands, and right leg. She had edema of the ankles and feet and dyspnea.
Laboratory investigations revealed hypochromic microcytic anemia with leukocytosis (22,600 uL; reference: 4,600-10,200 uL) and eosinophilia (9,492 uL; reference: 0-400 uL). Rheumatoid factor, ANA, ANCAs, troponin I (0.06 ng/dl; reference: 0-0.4 ng/mL) were negative. Brain naturetic peptide (BNP) was 404 pg/ml.
Chest x-ray showed cardiomegaly. Transthoracic echocardiography showed biventricular dilation and dysfunction. An estimated left ventricular ejection fraction of 31% with global hypokinesia of the left ventricle was noted; dysfunction was more severe in the anterior, anterolateral and inferolateral walls. Intermediate probability of pulmonary hypertension with an estimated systolic pulmonary artery pressure of 46mmHg was detected. A moderate pericardial effusion was present. A coronary computed tomographic angiography (CCTA) documented absence of coronary artery disease (CAD-RADS 0).
CMR findings
Given the patient’s age, biventricular dysfunction and pericardial effusion on echocardiogram, cardiovascular MRI was performed to further assess cardiac function and viability. The MRI confirmed a dilated left ventricle (LVIDd: 61 mm, LVEDV: 94.2 mL/m2, LVESV: 60.5 mL/m2), biventricular dysfunction (left ventricular EF 36%, right ventricular EF 31%) with a moderate pericardial effusion (Movies 1 and 2). Short axis T2 weighted images did not show edema (hyperintensity); the signal intensity ratio of myocardium over skeletal muscle was < 2.
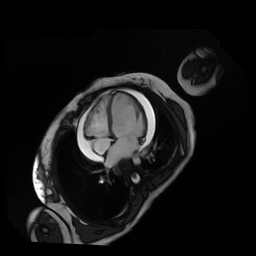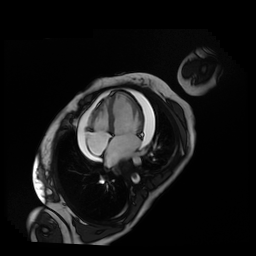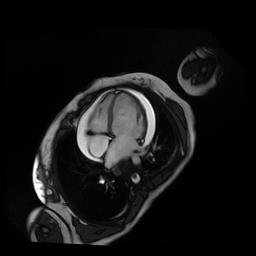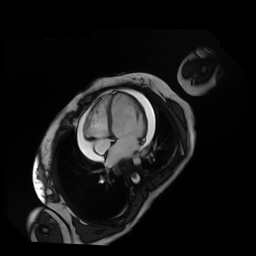
Movie 1
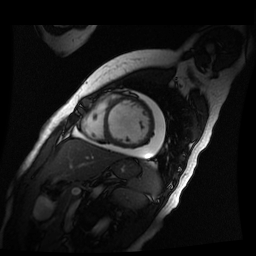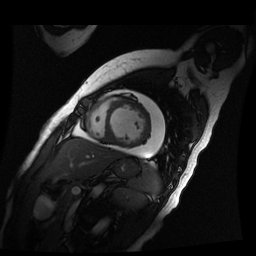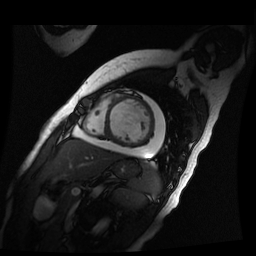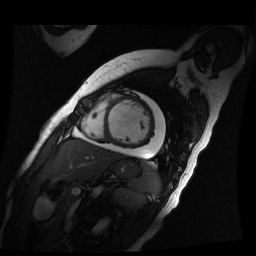
Movie 2
Inversion recovery-prepared gadolinium-enhanced T1-weighted images showed patchy, diffuse subendocardial delayed enhancement involving the basal anteroseptal, inferior, inferolateral and anterolateral walls, the mid anteroseptal, inferoseptal and inferior walls and the apical anterior, septal and inferior walls. [Images 1-3]
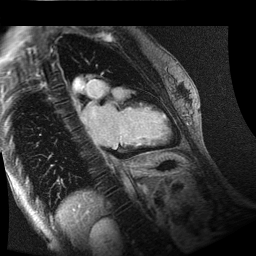
Image 1
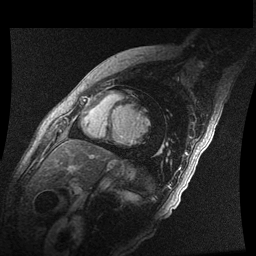
Image 2
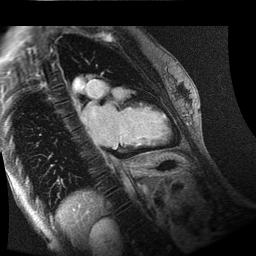
Image 3
Conclusion
Dilated cardiomyopathy in adults is most commonly caused by CAD and hypertension, although viral myocarditis, valvular disease and genetic predisposition may also play a role. In this case, some prominent left ventricular trabeculations were noted, but no diagnostic criteria for non-compaction was fulfilled (end-diastolic NC/C ratio 2.2 and trabeculated LV mass <20%). Left ventricular hypertrabeculation can be associated with dilated cardiomyopathy and heart failure.
The patient declined to undergo a confirmatory endomyocardial biopsy. Given the CMR findings, along with no evidence detected for infection, vasculitis, malignancy, drug induced hypersensitivity reactions or coronary artery disease, this case appears most consistent with eosinophilic myocarditis. The patient received treatment with oral prednisone 1 mg/kg/day with subsequent improvement in blood eosinophil count. The cardiac function has showed no recovery. The patient is currently being treated medically for heart failure.
Perspective
With cardiac damage first described by Loeffler in 1936, rates of cardiac involvement upwards of 80% were reported in early case series of hypereosinophilic syndrome (HES). In more recent studies, eosinophilia associated cardiac disease is present in about 20% of HES patients and one third of patients with eosinophilic granulomatosis and polyangiitis.1-4
Eosinophilic myocarditis is one of the most fatal complications of hypereosinophilia (HES) and is the cause of mortality in 48-75% of patients with HES, however it has been rarely reported in the literature.5-7
Patients with eosinophilic myocarditis (EM) may present with subacute heart failure, may mimic an acute coronary syndrome and more rarely, present with cardiogenic shock. EM is often suspected when patients with acute chest symptoms fail to improve with ACS, and cardiac catheterization reveals normal coronary arteries. 6,8
Recent reports support the use of CMR as an adjunct to diagnosis through the visualization of myocyte infiltration, inflammation, and edema, although these changes are not always present. In cardiovascular MRI, eosinophilic myocarditis is characterized by extensive myocardial hyperintensity on T-2 weighted imaging with subendocardial delayed enhancement. Mesocardial and epicardial delayed enhancement have also been reported. The extent of delayed enhancement is inversely proportional to LV ejection fraction. Cardiac CT provides little information beyond assessing for concomitant coronary artery disease. 7-10
View the entire case on Cloud CMR: https://www.cloudcmr.com/2357-4003-2358-4950/
References
4. Cheung CC, Constantine M, Ahmadi A, Shiau C, Chen LYC. Eosinophilic Myocarditis: A Concise Review for Clinicians. The American Journal of the Medical Sciences, http://dx.doi.org/10.1016/j.amjms.2017.04.002
5. Ginsberg F, Parrillo JE. Eosinophilic myocarditis. Heart Fail Clin 2005; 1: 419-29.
6. Al Ali AM, Straatman LP, Allard MF, Ignaszewski AP. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol 2006; 22: 1233-37.
Associate Editor: Robert D. Tunks, MD





