Frank J. Raucci Jr. MD PhD1, Patrick R. Holmes MD1, Brett A. Mettler MD2, Jason T. Christensen MD1
Division of Pediatric Cardiology1 and Cardiothoracic Surgery2 Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tennessee
Clinical History
A 6 year old male presented to the emergency department with a 2 week history of intermittent fever, fatigue, anorexia, and weight loss. His past medical history is significant for a history of refractory Kawasaki Disease diagnosed 1 year prior to presentation, which required two infusions of high-dose intravenous immunoglobulins, but had no coronary artery ectasia. He was followed by an outside cardiologist and no effusion or cyst had been noted during follow-up. He saw his primary care physician at the start of the current illness when he developed a scarletina rash and was diagnosed with streptococcal pharyngitis. His mother noted that he failed to improve on bicillin and developed a worsening nighttime cough. He was seen multiple times by his primary care physician and had a work-up notable for elevated inflammatory markers [erythrocyte sedimentation rate of 120 mm/hr (normal 1 – 33 mm/hr), platelet concentration of 800 x103/µL (normal 150 – 400 x103/µL), and c-reactive protein concentration of 9 mg/L (normal 0.1 – 1.0 mg/L)] and viral panel positive for adenovirus. A chest x-ray was obtained demonstrating marked cardiomegaly, and he was sent to the emergency department for further evaluation. Although he denied chest pain, dyspnea, or orthopnea, an echocardiogram was performed given the x-ray finding and history as well as a prominent subxiphoid impulse and a faint friction rub heard on exam. Initial echocardiogram demonstrated a moderate-sized posterior pericardial effusion and a large, well-circumscribed, heterogeneous mass in the pericardial space adjacent to the left ventricular wall. There was rightward shift of the heart within the pericardium due to mass effect but no significant evidence of pericardial tamponade (Movies 1 and 2, Image 1).

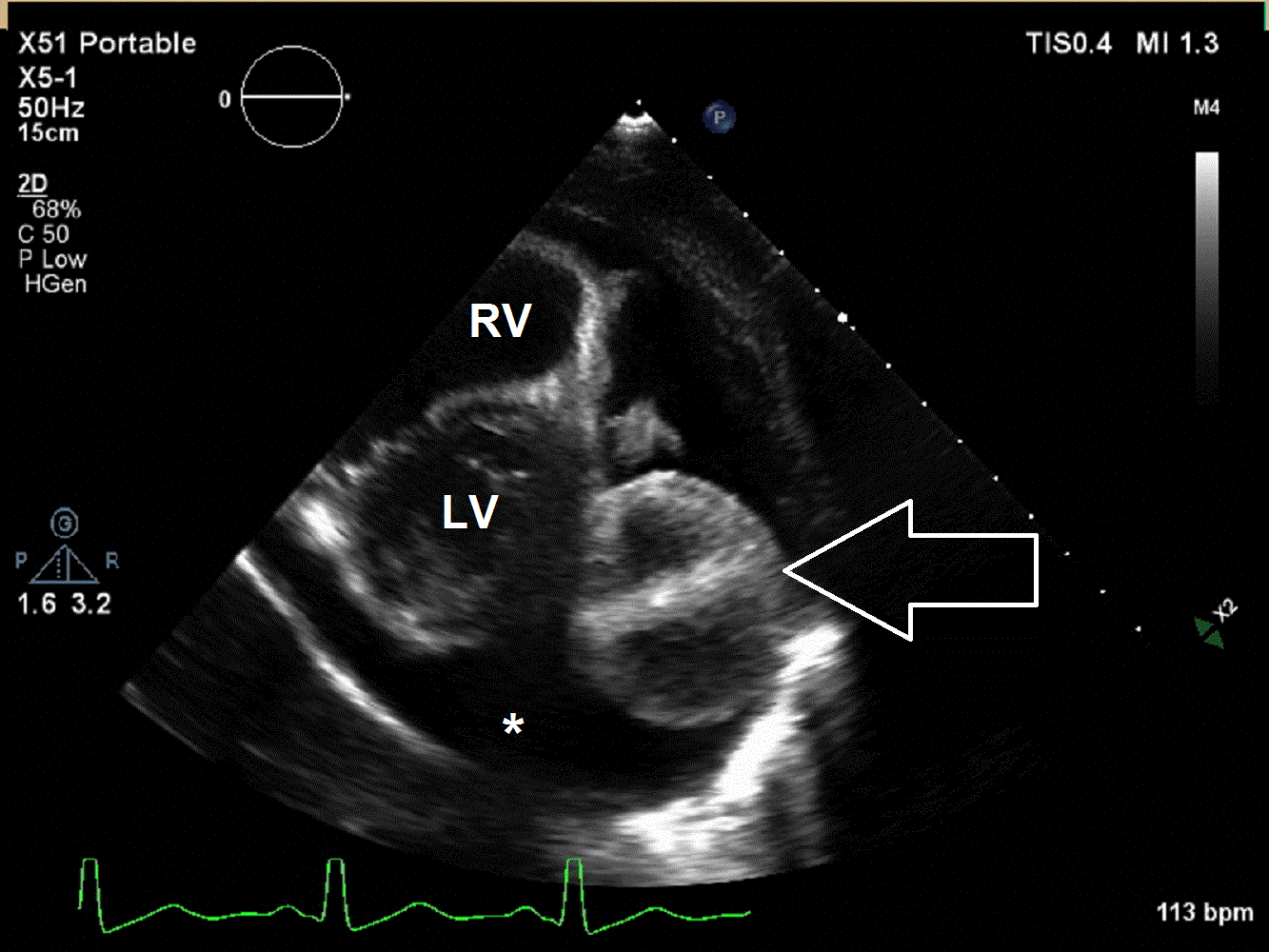
Movie 1 and 2, Image 1: Subcostal coronal and apical four chamber transthoracic echocardiogram. Large lobulated pericardial mass (white arrow) with an associated large pericardial effusion (*) and normal bi-ventricular size and systolic function.
CMR Findings
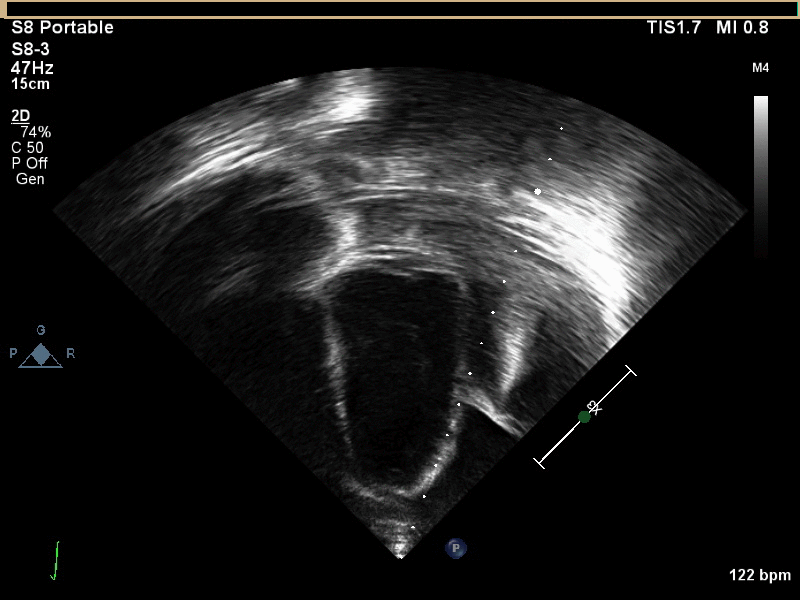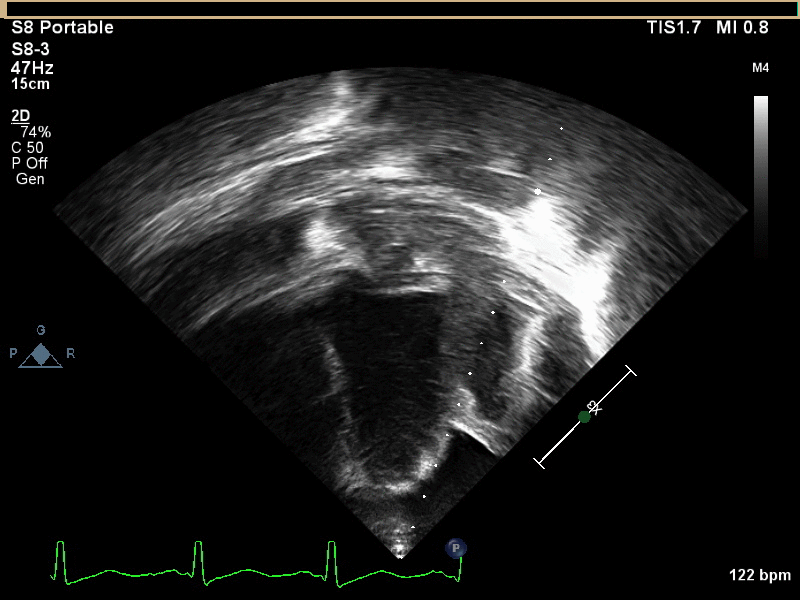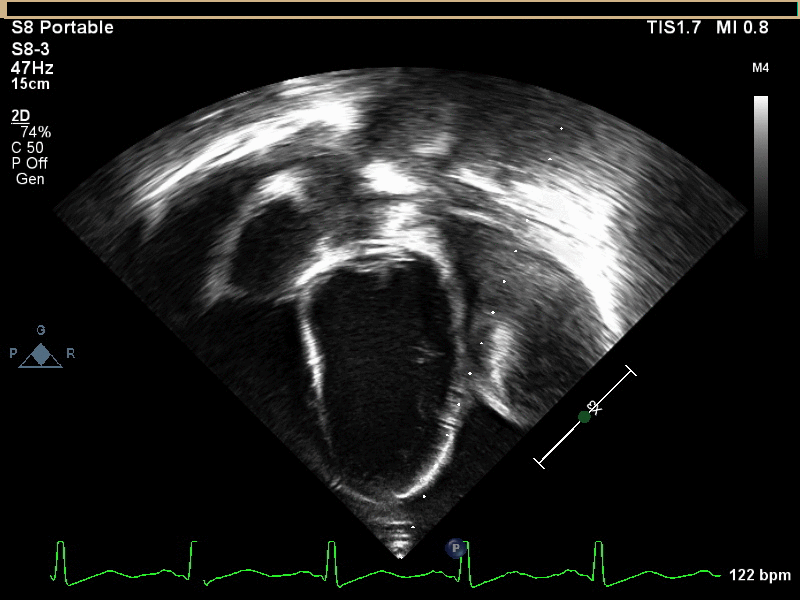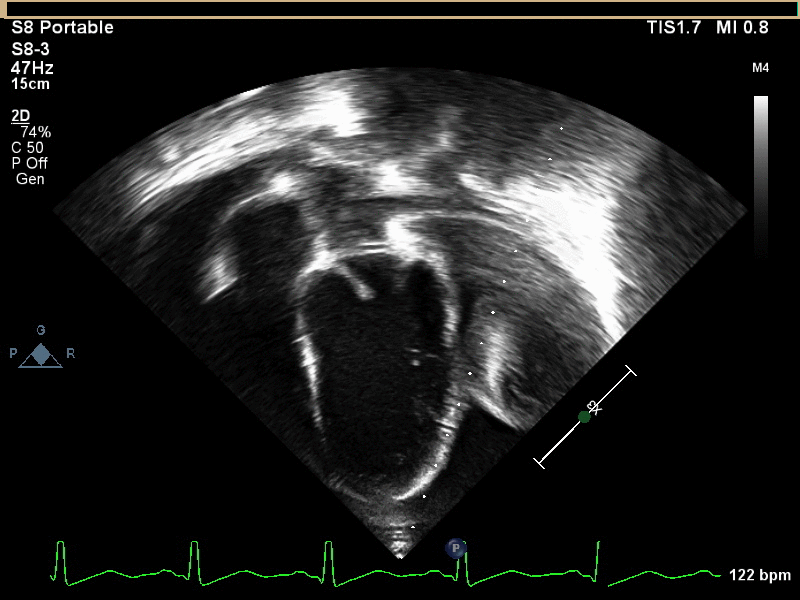
CMR with multiple sequences was performed in order to aid in the diagnosis of the mass. Using established CMR diagnostic criteria [1] and a stepwise scanning approach, we proceeded to develop a differential diagnosis for the mass. Pre-contrast T1 weighted turbospin echo imaging with and without fat saturation demonstrated a lobulated mass adjacent the pericardium. The mass was hyperintense with and without fat saturation (Movie 3 and 4, Image 2 and 3), excluding lipoma. Pre-contrast T2 weighted imaging with fat saturation (Movie 5, Image 4) demonstrated hypointensity of the pericardial fluid and in the mass’s center, suggesting the contents of the mass and pericardial fluid represented decomposition products of blood. First pass perfusion (FPP) imaging demonstrated absence of contrast in the core of the mass with rim enhancement simultaneous with pericardial enhancement (Movie 6, Image 5), suggesting pericardial origin of the outer surface of the mass and excluding a vascular malformation as a diagnosis. On delayed enhancement imaging (Movie 7), the pericardium and external rind of the mass enhanced, excluding a diagnosis of fibroma. On respiratory navigated 3D steady state free precession (SSFP) imaging, the central fluid was isointense with blood and pericardial connections with septae to the epicardial service could be seen (Movie 8). Taken together, this study suggested a pericardial cyst with intracavitary hemorrhagic products and hemorrhagic pericardial effusion.
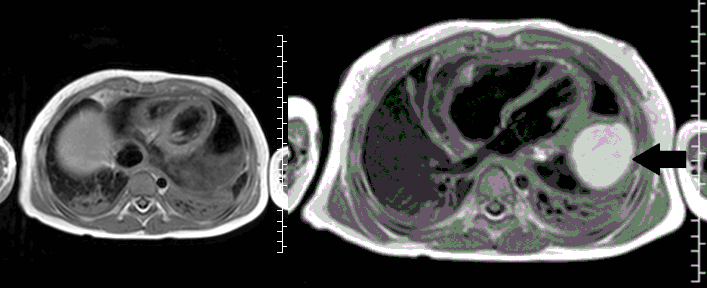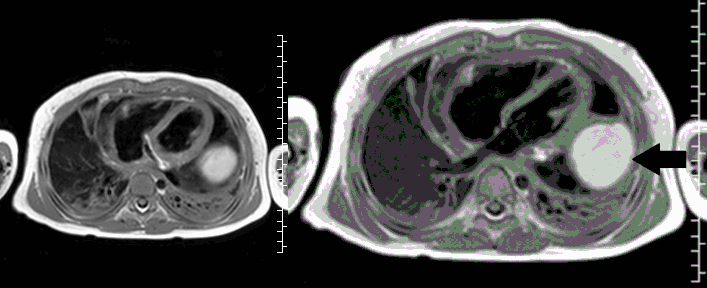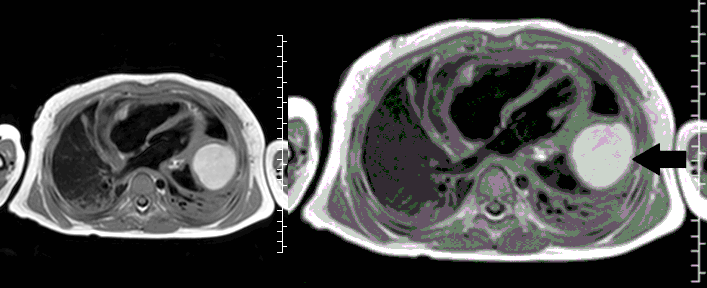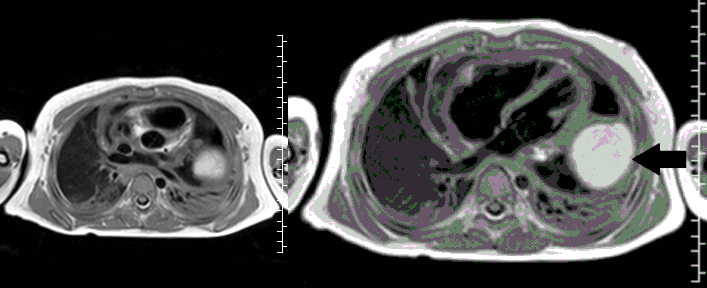
Movie 3, Image 2: Pre-contrast T1-weighted without fat saturation shows a hyperintense lobulated pericardial mass (black arrow).

Movie 4, Image 3: Pre-contrast T1-weighted with fat saturation shows a hyperintense lobulated pericardial mass (white arrow).
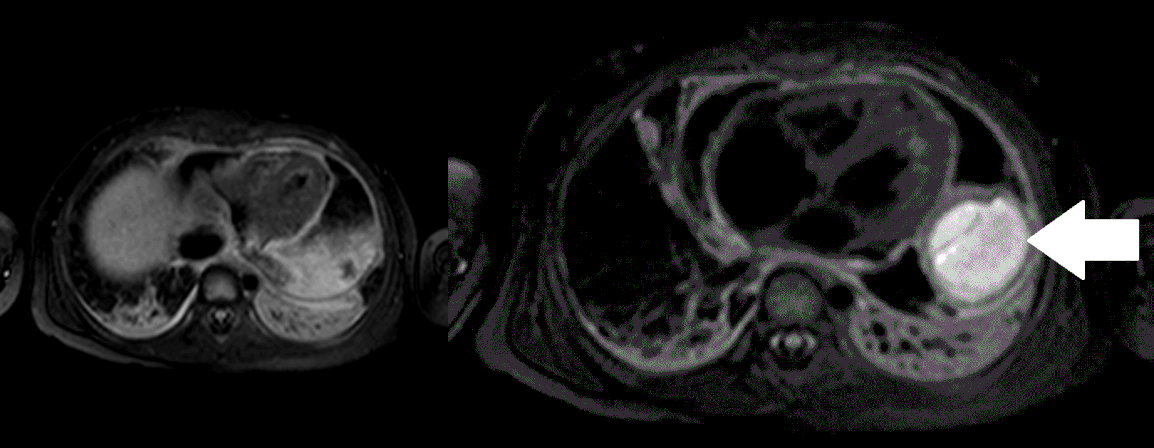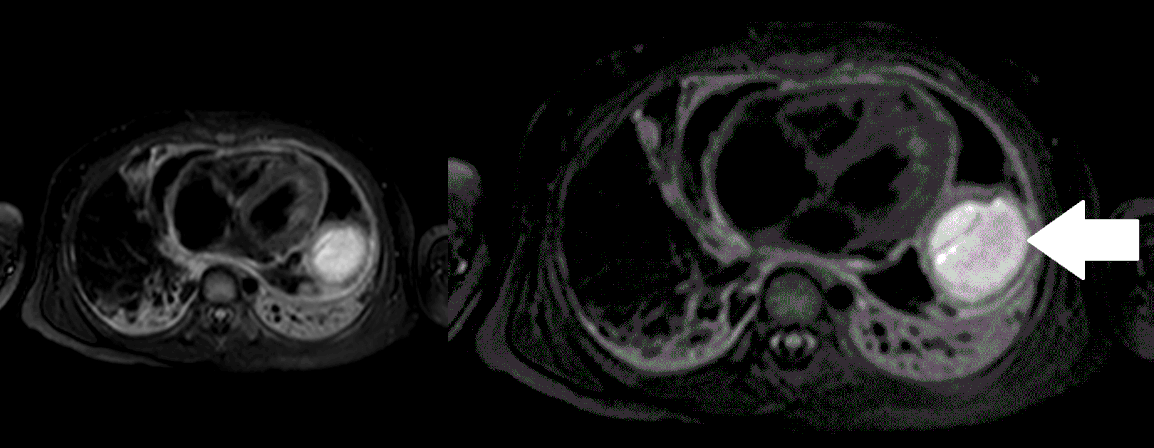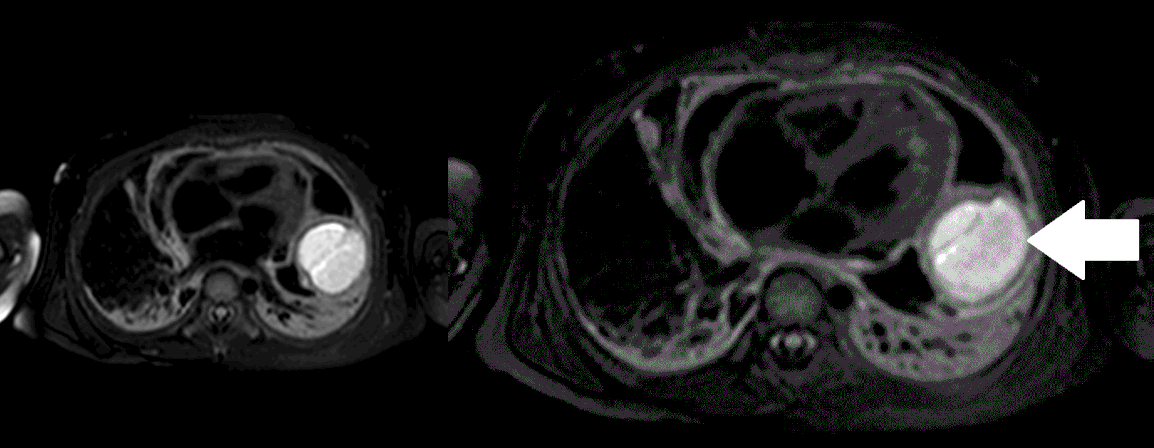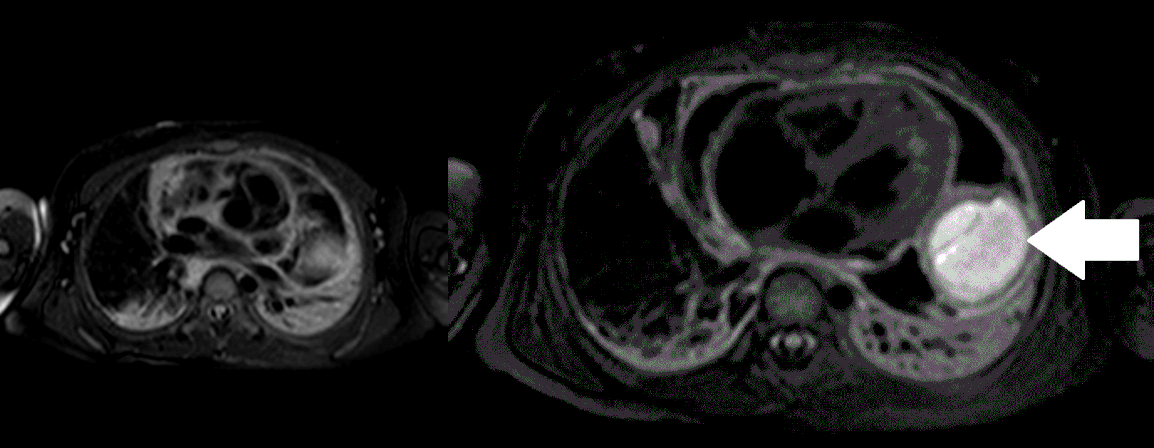
Movie 5, Image 4: Pre-contrast T2-weighted with fat saturation shows hypointensity of the pericardial fluid and in the mass’s center.

Movie 6, Image 5: First pass perfusion demonstrates absence of contrast in the core of the mass (*) with rim enhancement of the mass (white arrow outline) simultaneous with pericardial enhancement (solid white arrow).
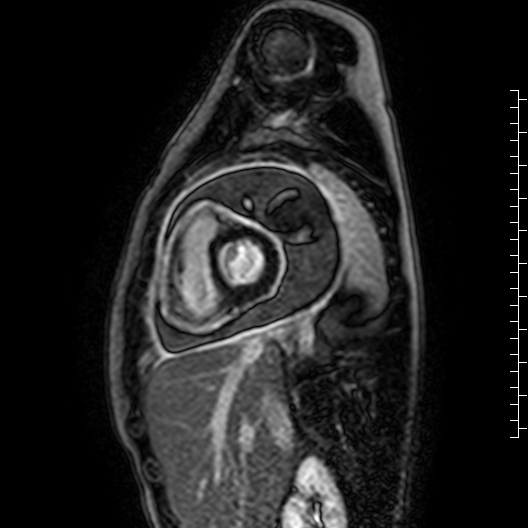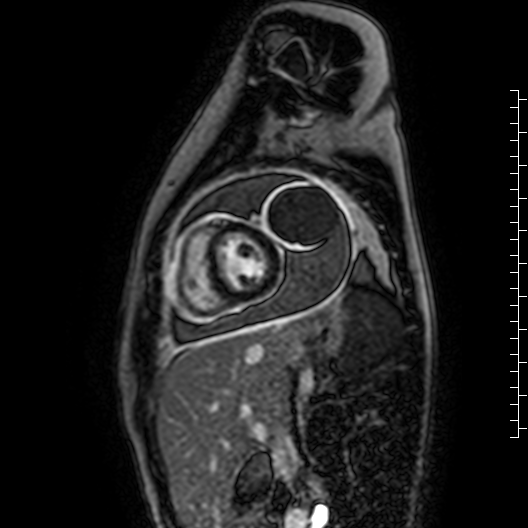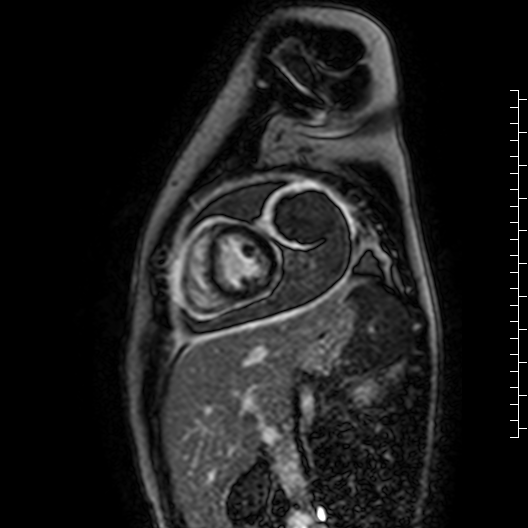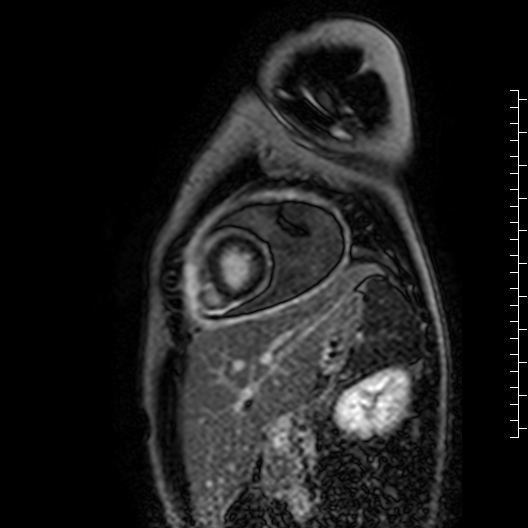
Movie 7: Delayed enhancement images: Phase-sensitive inversion recover (PSIR) images demonstrating enhancement only of pericardium and external rind of the cyst.
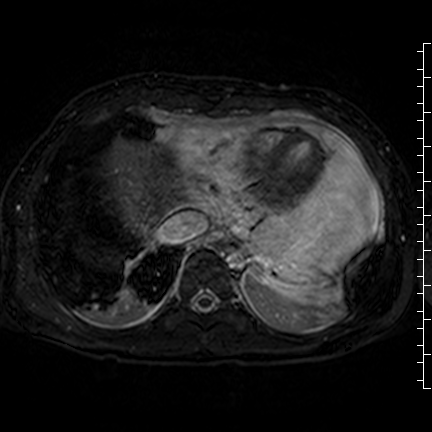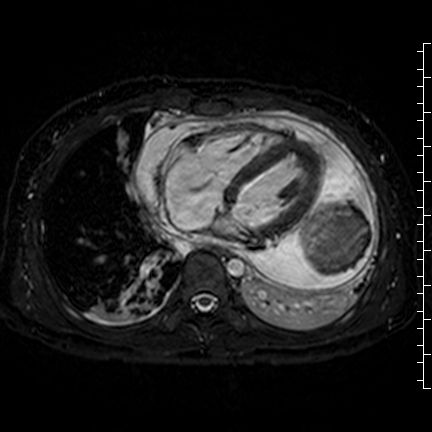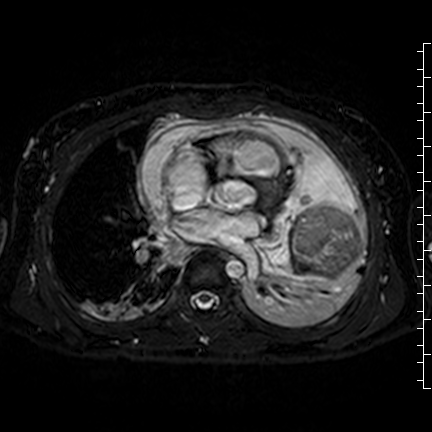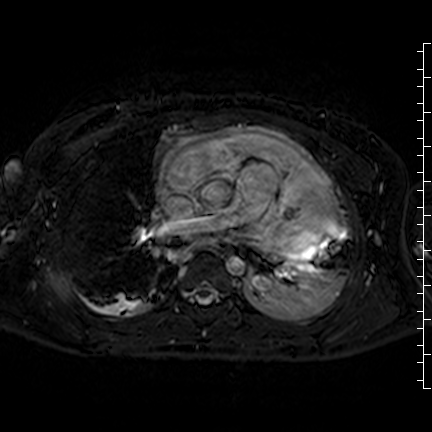
Movie 8: Three dimensional steady state free precession shows the central fluid of the mass was isointense with blood and pericardial connections with septae to the epicardial surface.
Conclusion
Cardiac MRI findings were most consistent with pericardial cyst complicated by intracystic hemorrhage and hemorrhagic pericardial effusion. Surgical resection was deemed the best course of action. On operative inspection, the pericardium was noted to be very thickened with a rind, which required decortication (Image 6). A pericardial stalk connected the pericardium to the mass was identified originating posterior to the left ventricle and a 4 cm x 4 cm fluid-filled mass was removed. Pathological examination confirmed a hemorrhagic pericardial cyst.
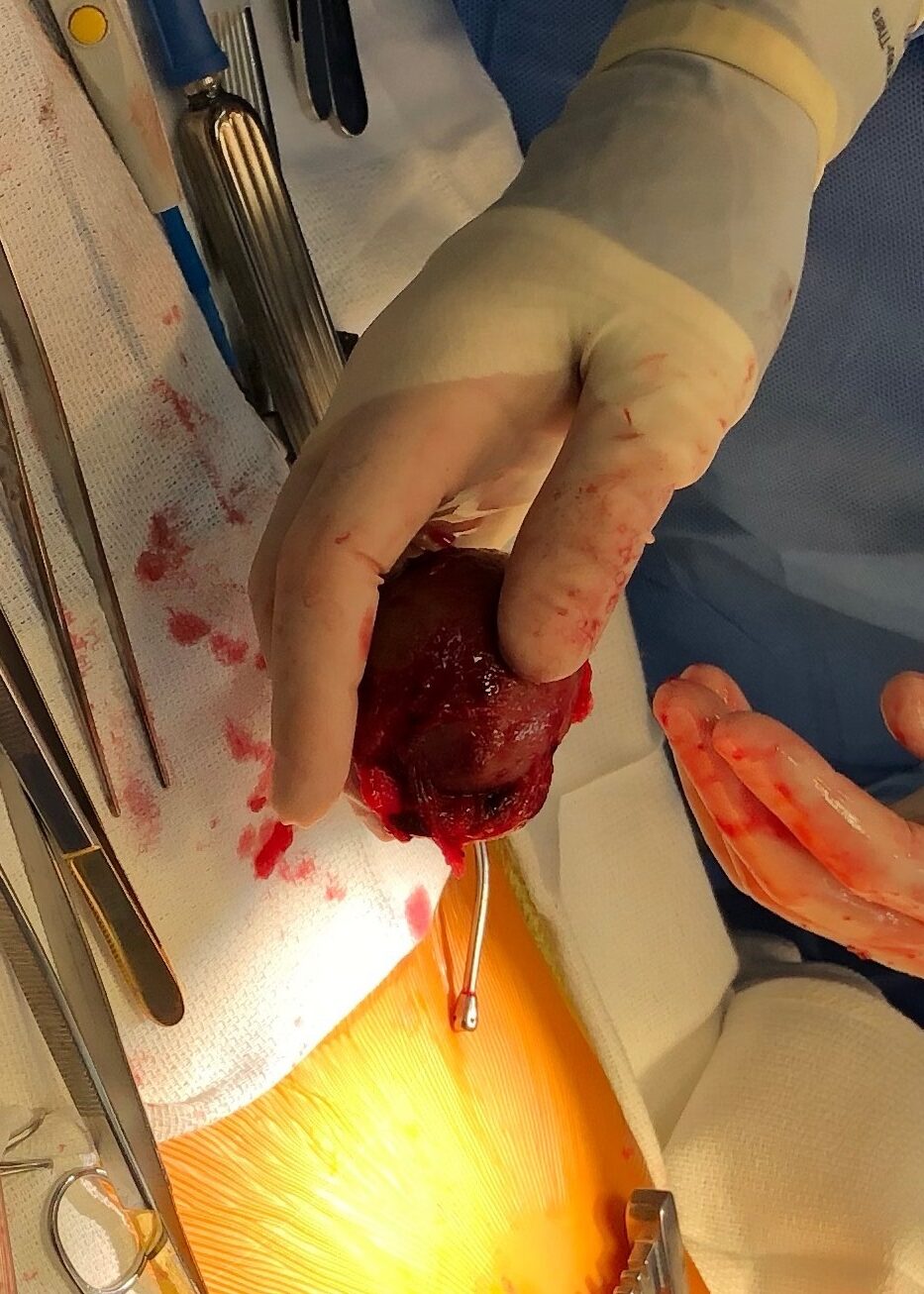
Image 6: Intraoperative appearance of the pericardial mass.
Perspective
Pericardial cysts are a relatively rare entity, with an estimated incidence of 1 in 100,000 [2]. They are believed to develop secondary to failure of fusion of one of the mesenchymal lacunae that form the pericardial sac and are lined with either endothelium or mesothelium. Pericardial cysts are usually benign and when symptoms occur they are usually due to impingement on surrounding structures. Symptomatic patients may present with nausea, dyspnea, or atypical chest pain. Intracystic hemorrhage is rare, however rupture of these cysts can lead to rapid accumulation of blood in the pericardium with resultant tamponade physiology [3, 4]. While it is unclear in this case if the pericardial cyst or hemorrhage was related to the patient’s acute infection or remote complication of Kawasaki Disease, there have been rare reports of polyserositis in the acute Kawasaki disease leading to severe complications [5, 6]. The history of Kawasaki disease may also represent a marker for a genetic susceptibility to robust inflammatory reactions in the setting of infection. Recently, a tumor necrosis factor α (TNFα)-mediated mechanism for pericardial effusion has been proposed as a distinct pathway separate from that of coronary ectasia formation in Kawasaki disease [7]. TNFα may lead to hyperpermeability of the endothelium, predisposing to serum protein loss and potentially hemorrhage. This may represent a possible link for the inflammation and hemorrhage seen in the presented case.
Click here to view the case on CloudCMR.
1. Beroukhim, R.S., et al., Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol, 2011. 58(10): p. 1044-54.
2. Patel, J., et al., Pericardial cyst: case reports and a literature review. Echocardiography, 2004. 21(3): p. 269-72.
3. Marigliano, A., E.M. Cirio, and R. Versace, [Pericardial cyst with intracystic hemorrhage. A case report and review of the literature]. G Ital Cardiol (Rome), 2010. 11(6): p. 493-7.
4. Temizkan, V., et al., Hemorrhage into a pericardial cyst and associated right ventricular compression after blunt chest trauma. Ann Thorac Surg, 2010. 89(4): p. 1292-5.
5. Dahlem, P.G., et al., Pulse methylprednisolone therapy for impending cardiac tamponade in immunoglobulin-resistant Kawasaki disease. Intensive Care Med, 1999. 25(10): p. 1137-9.
6. Ozdogu, H. and C. Boga, Fatal cardiac tamponade in a patient with Kawasaki disease. Heart Lung, 2005. 34(4): p. 257-9.
7. Okada, S., et al., Acute pericardial effusion representing the TNF-alpha-mediated severe inflammation but not the coronary artery outcome of Kawasaki disease. Scand J Rheumatol, 2015. 44(3): p. 247-52.
Case prepared by:
Jason N. Johnson MD MHS
Associate Editor SCMR Case of the Week
Le Bonheur Children’s Hospital
University of Tennessee Health Science Center





