Sylvia Krupickova1,2, Ricardo Wage2, Mary Lane3, Olivier Ghez1, Dudley J Pennell2, Inga Voges1,2
1. Department of Paediatric Cardiology, Royal Brompton Hospital, London, UK
2. Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, London, UK
3. Department of Anaesthesiology, Royal Brompton Hospital, London, UK
Clinical History
A four-year old patient with pulmonary atresia and ventricular septal defect (PA/VSD) after surgical repair with Contegra conduit (bovine jugular vein graft, Medtronic, Minneapolis, MN) at the age of 2 years underwent repeated CMR scan under general anesthesia for evaluation of aortic regurgitation and assessment of left ventricular volumes. CMR showed significant aortic regurgitation through a perforation in the right coronary cusp with mild left ventricular dilatation and mildly impaired left ventricular systolic function. Surprisingly, severe stenosis of the pulmonary conduit due to a mobile mass with severe dilatation and severe dysfunction of the right ventricle was recognized. These findings were not seen on echocardiogram performed 2 months before and were suspicious of infectious endocarditis (IE).
Subsequently, the patient was found to have a recent history of pneumonia and fever treated with oral antibiotics. He recovered well at first before becoming more tired and having increased work of breathing at the time of the electively planned CMR. His blood results showed hypochromic anaemia, mild neutrophilia and monocytosis and only mildly raised CRP. Blood cultures were positive for Streptococcus cristatus. No other clinical signs of IE have been noticed and early surgical replacement of the right ventricular outflow tract confirmed a vegetation within the conduit. The aortic valve perforation was repaired with a patch of mitral valve homograft during the same surgical procedure. The patient is clinically well since surgery.
CMR Findings
CMR demonstrated at least moderate aortic regurgitation with the diastolic jet directed towards the ventricular septal defect (VSD) patch (Movie 1 and 2). This was caused by a perforation of the right coronary cusp from previous surgical injury (Figure 1). The left ventricle was mildly dilated (end-diastolic volume 106 ml/m2) with mildly reduced ejection fraction (EF 56%) and increased left ventricular mass index (74 g/m2).
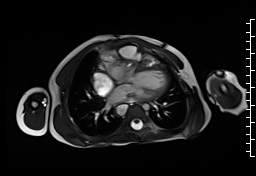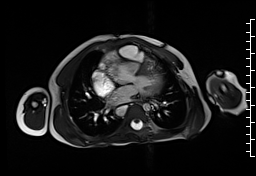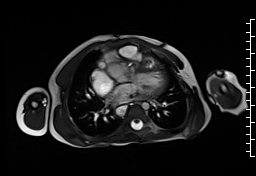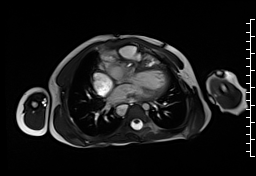
Movie 1: Axial cine SSFP with aortic valve insufficiency directed toward the ventricular septal patch.
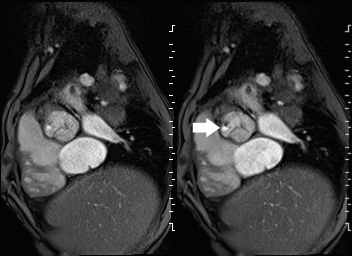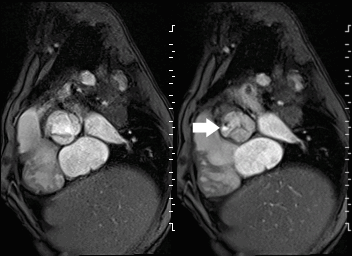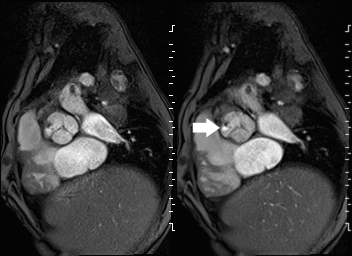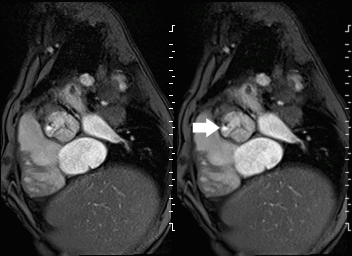
Movie 2 and Image 1: En face aortic valve cine GRE with a perforation (white arrow) in the right coronary cusp.
The right ventricle was severely dilated (end-diastolic volume 166 ml/m2, ratio of the end-diastolic volumes of the right and left ventricles RV EDV:LV EDV = 1.6:1) with severe systolic dysfunction (EF 23%) (Movie 3). There was moderate right ventricular hypertrophy with marked trabeculations. Right ventricular contraction was prolonged compared to the left ventricle with end-systolic and diastolic flattening of the interventricular septum (Movie 4).
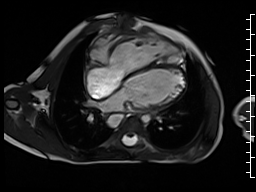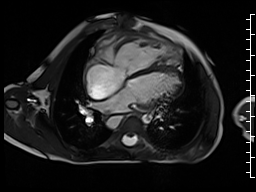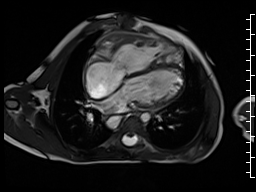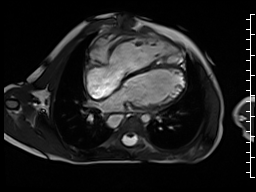
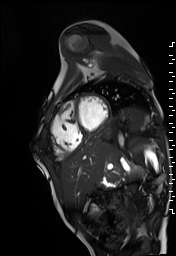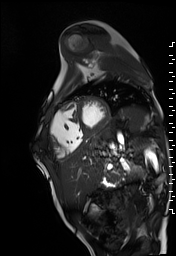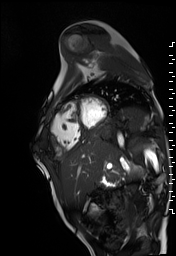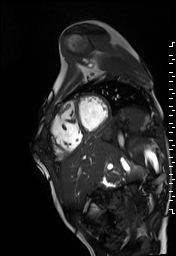
Movie 3 and 4: Four chamber and short axis cine SSFP with severe right ventricular chamber dilation and systolic dysfunction, and diastolic flattening of the interventricular septum.
A mobile mass (dimensions 8 x 11 mm) at the level of the right ventricular outflow tract conduit was illustrated, causing severe conduit stenosis (Movie 5-7, Image 2). The mass showed similar intensity to the myocardium on the gradient echo cine images. Some cine images showed additional hypointense areas especially in the left pulmonary artery. On early gadolinium enhancement CMR the mass appeared with low signal intensity (Image 3), whereas late gadolinium enhancement (LGE) imaging revealed rim enhancement of the mass (Image 4). Time-resolved 3D contrast-enhanced magnetic resonance angiography (CE-MRA) did not detect major lung perfusion defect (Movie 8) and did not confirm any masses in the main pulmonary artery and branches suggesting the hypointense areas on cine images being artefacts.
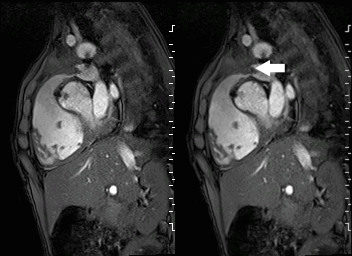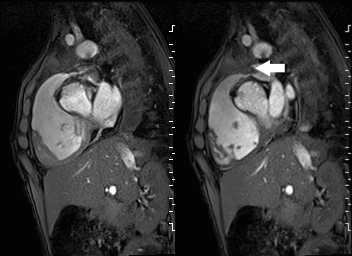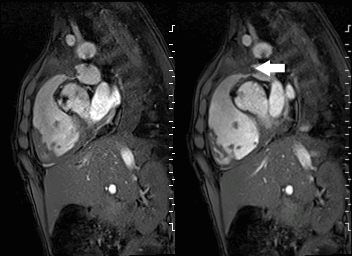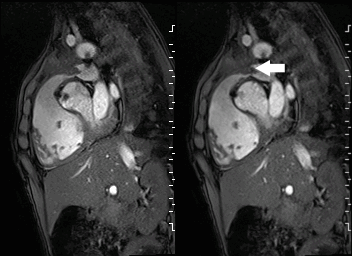
Movie 5 and Image 2: Cine GRE with a mobile mass (white arrow) in the right ventricular outflow tract.
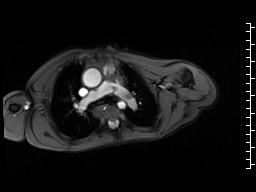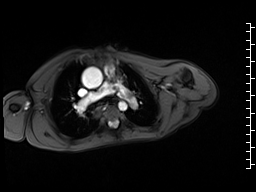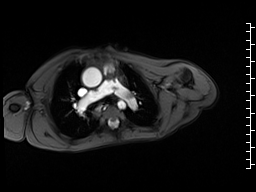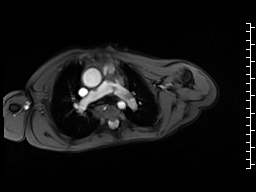
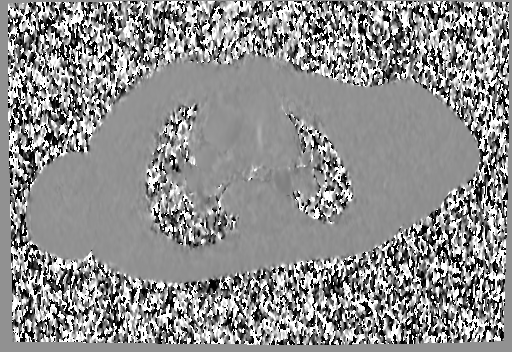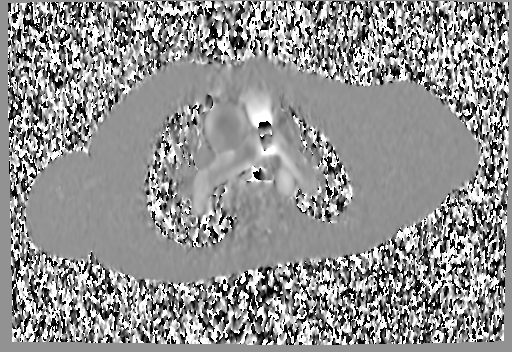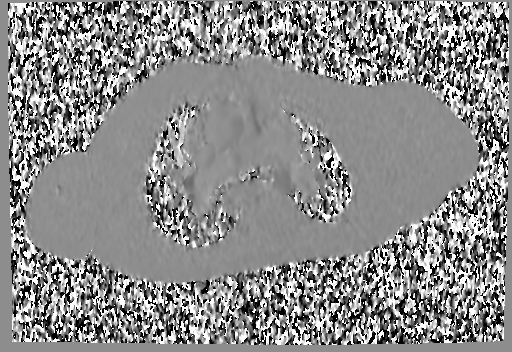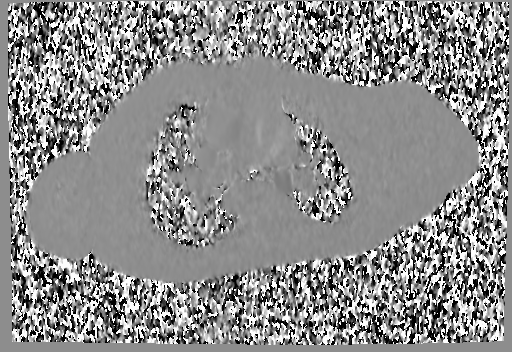
Movie 6 and 7: Cine GRE and phase contrast of the branch pulmonary arteries with a mobile mass in the right ventricular outflow tract and severe conduit stenosis.
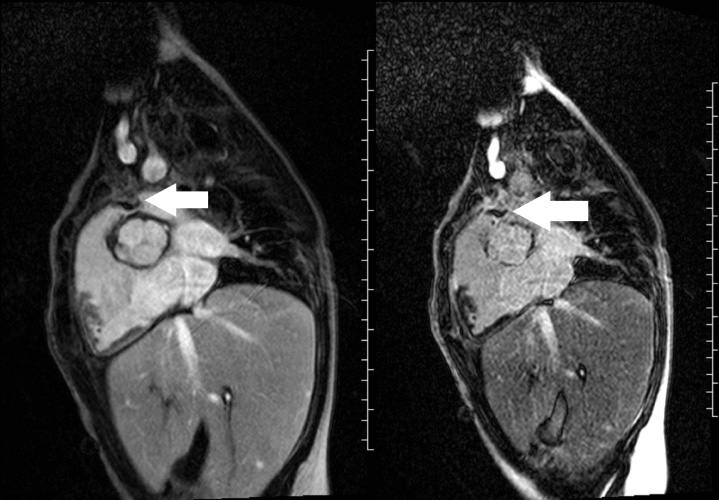
Image 3 and 4: Right ventricular outflow tract view early gadolinium enhancement (left image) revealed the mass to have low signal intensity (white arrow), and the late gadolinium enhancement (right image) revealed the mass to have rim enhancement (white arrow).
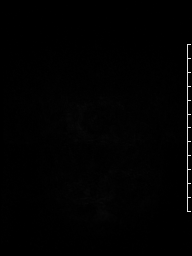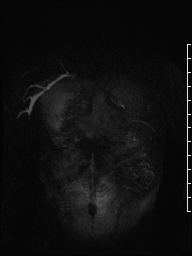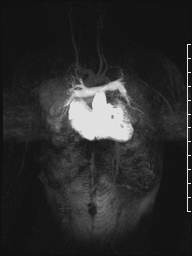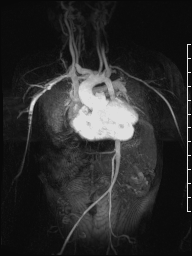
Movie 8: Time-resolved 3D contrast-enhanced magnetic resonance angiography did not detect major lung perfusion defect.
LGE imaging also detected areas of fibrosis at surgical sites and beyond (VSD patch, right ventricular outflow tract, mid-ventricular septum extending from the VSD patch; Image 5 and 6).
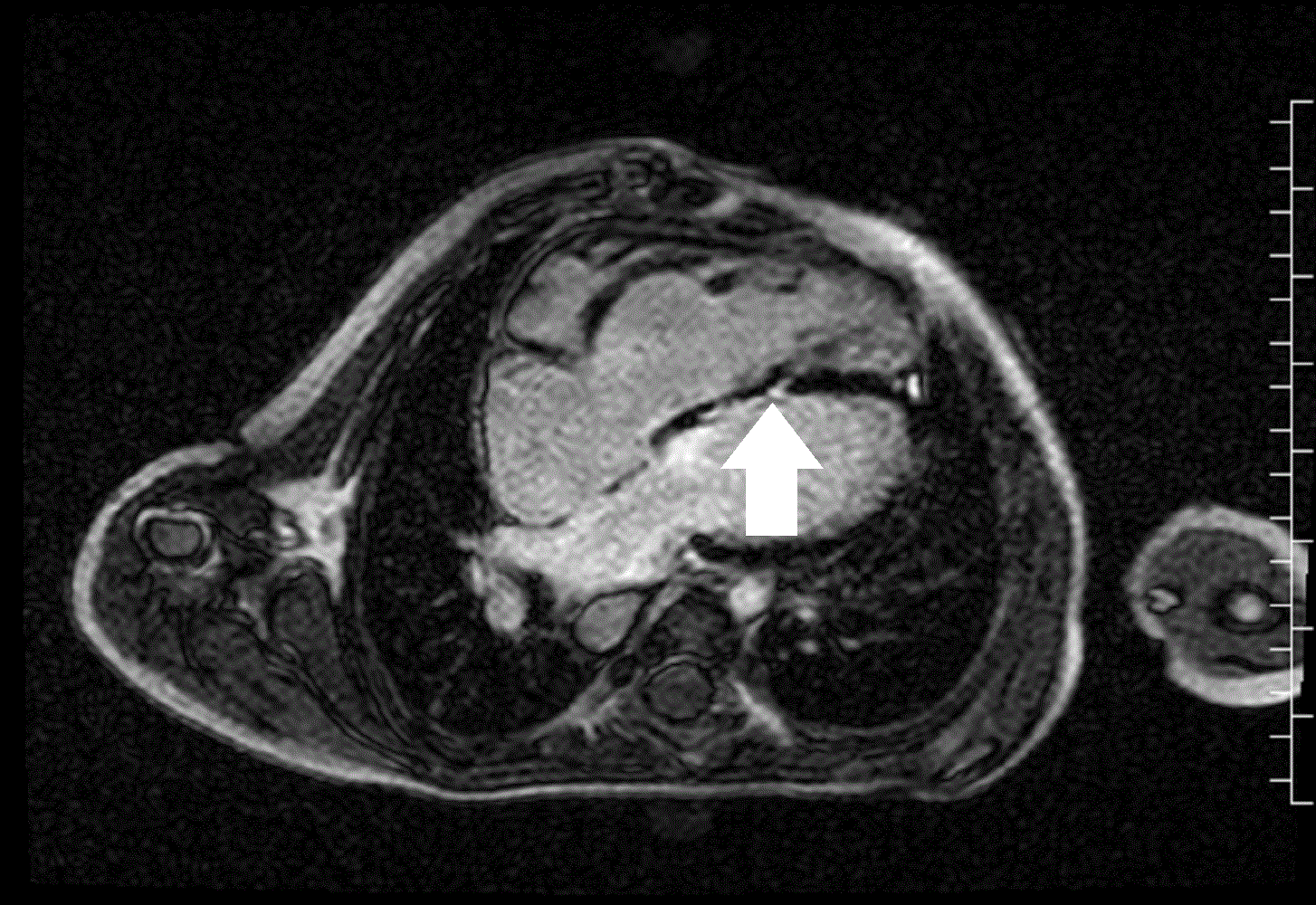
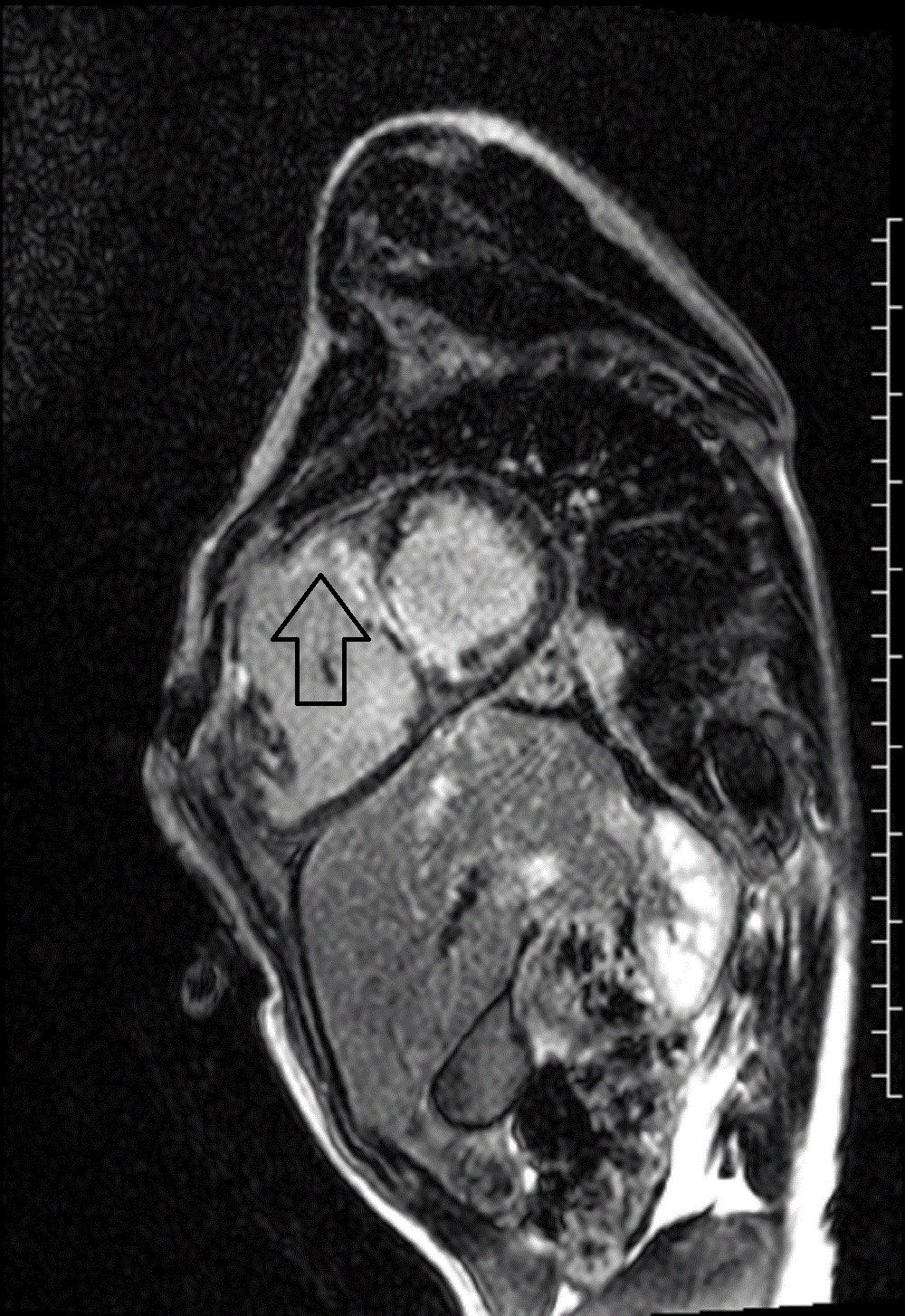
Image 5 and 6: Late gadolinium enhancement four chamber (left image) and short axis (right image) with fibrosis in the midventricular septum (white arrow) and right ventricular outflow tract (black arrow).
Following CMR diagnosis, CT was performed in order to explore lung changes. No emboli were found and a single mass was confirmed within the conduit. Multiplanar reconstruction showed the mass in the conduit (Image 7). The pulmonary vasculature was easily seen by CT as well (Image 8).
Echocardiogram performed on the day of CMR scan showed also a mass at the valve within the conduit with significantly increased velocity in comparison to the echocardiogram performed 2 months ago before the acute illness. However, the imaging resolution and tissue characterisation were much better on the CMR scan (Image 9).
Patient underwent surgical replacement of the conduit and the explanted conduit with vegetation is shown in Image 10. The aortic valve was repaired with mitral valve homograft patch.
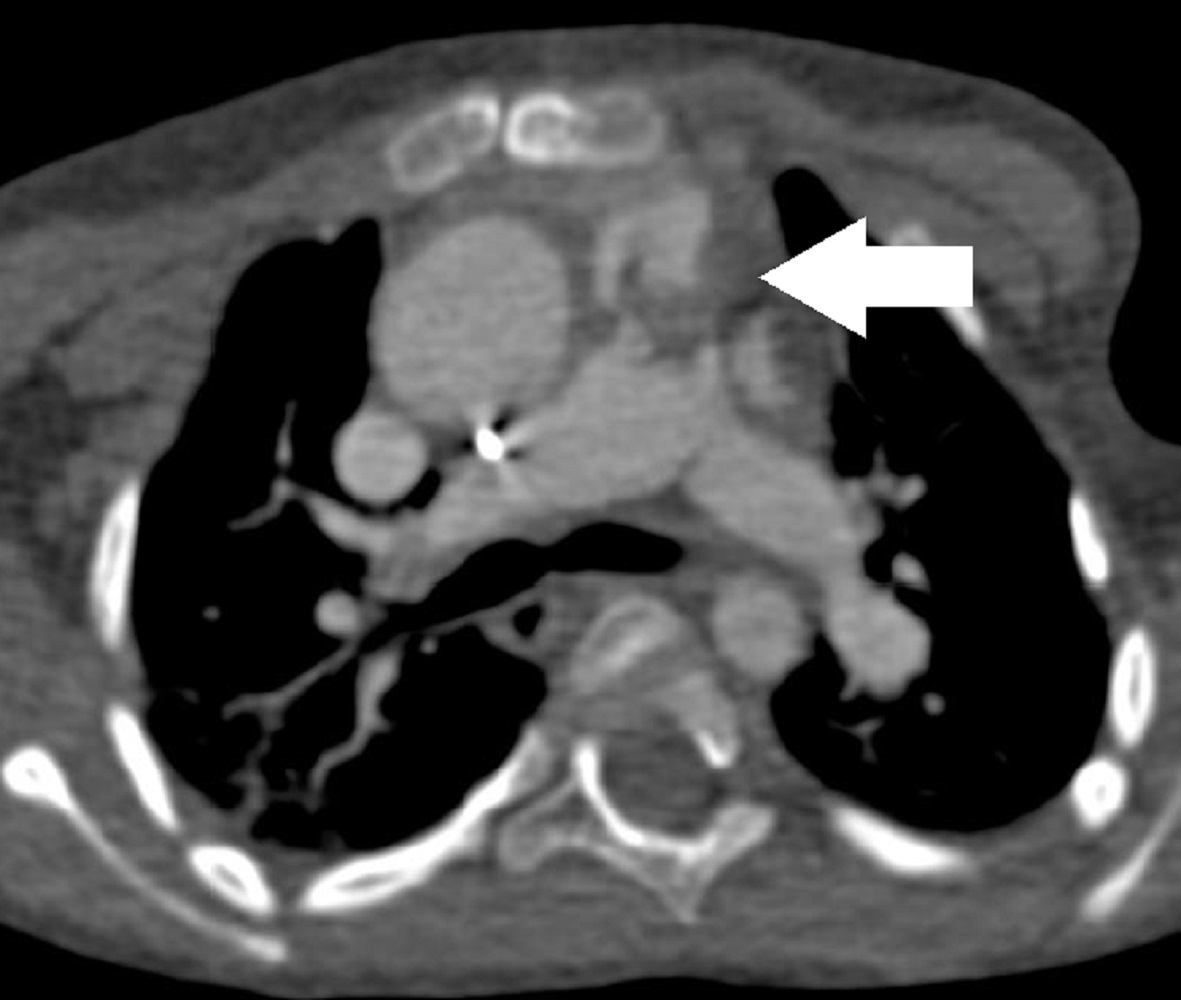
Image 7: CT multiplanar reconstruction of the mass (white arrow) in the right ventricle to pulmonary artery conduit.
Image 8: CT in coronal plane showing normal pulmonary vasculature.
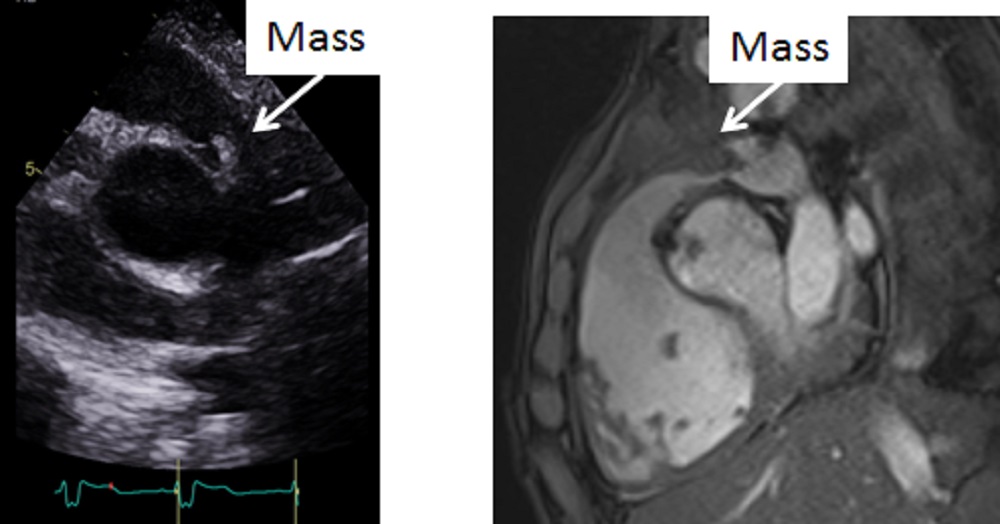
Image 9: Side by side comparison of the transthoracic echocardiogram versus the CMR showing the mass in the right ventricular outflow tract better delineated on the CMR.
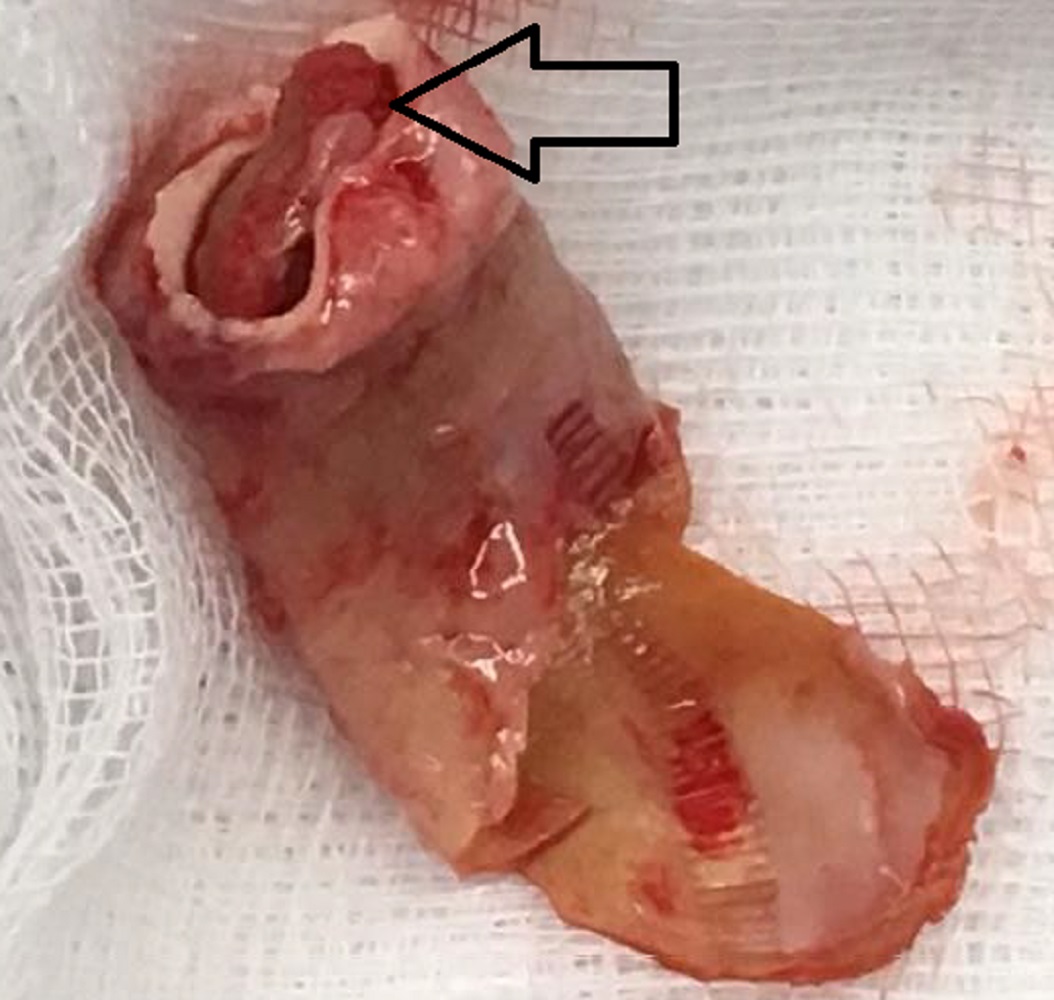
Image 10: The explanted right ventricle to pulmonary artery conduit with the vegetation (black arrow) present.
Conclusion
Larger vegetations together with haemodynamic implications can be diagnosed with CMR. We reported a patient with right ventricular outflow tract conduit in whom CMR was crucial for the correct diagnosis and lead to successful surgical conduit replacement.
Perspective
The incidence of PA/VSD is estimated at 6.1 per 100,000 live births (1). It is thought to be an extreme form of tetralogy of Fallot with typically large perimembranous ventricular septal defect, overriding aorta and atresia of the pulmonary outflow tract causing hypertrophy of the right ventricle. Three types of pulmonary blood supply are recognized: 1.) pulmonary vascular supply via native pulmonary branches through patent arterial duct, 2.) supply via major aortopulmonary collaterals (MAPCAs) with absent native pulmonary branches, 3.) combination of both sources (2).
Multistage repair is usually required although one-stage repair has also been performed in some cases. The most severe forms are typically palliated with a Blalock-Thomas-Taussig shunt.
The overall estimated survival rate of PA/VSD at 1, 10 and 30 years of age is 73, 60 and 53%, respectively, and no difference between patients with or without MAPCAs has been shown. However, in patients who undergo complete repair, the Kaplan-Meier estimated survival rate is much better: 93% at 1 year and 91% from the second year on (1).
CMR has been established as a crucial modality for longitudinal comprehensive follow up assessment of patients after PA/VSD repair (3,4). It provides information about location and severity of right ventricular outflow tract and pulmonary artery obstruction, branch pulmonary artery size and flow distribution, residual atrial and ventricular septal defects, ratio of the pulmonary and systemic flow (Qp/Qs) and allows quantification of pulmonary regurgitation and ventricular parameters including right ventricular volumes and ejection fraction (3-5). The anatomical and functional data can be obtained using cine and phase-contrast, flow imaging as well as 3D data acquisitions (CE-MRA and 3DSSFP images). Late gadolinum enhancement is used to visualize areas of fibrosis.
In addition, CMR plays an important role in decision making for pulmonary valve replacement. It is now common practice to time the pulmonary valve replacement not solely according to the patients’ symptoms, but to take the right ventricular volume and function into consideration. An indexed end-diastolic right ventricular volume of 150-170 ml/m2 and an end-systolic of 80 ml/m2 have been shown to predict normalization of right ventricular size after pulmonary valve replacement (6).
IE is a serious potentially lethal complication of patients with congenital heart disease. The risk of IE estimated from a large population-based study in children with congenital heart disease up to 18 years of age is 6.1/1000 children, being the highest in cyanotic heart disease (7). Recently the type of conduit from right ventricle to pulmonary artery has been recognized as an important risk factor of IE; bovine jugular vein grafts were found to be a significant risk factor for conduit endocarditis when compared to cryopreserved homograft (8).
Vegetations are usually small, thin and mobile structures and transoesophageal echocardiogram is considered as a gold standard method for imaging them.
We presented a case where diagnosis of IE with a less mobile larger vegetation was established incidentally by an elective CMR scan and this was fundamental for the patient’s outcome.
Click here to view case on CloudCMR
References
1. Kaskinen AK, Happonen JH, Mattila IP, Pitkänen OM. Long-term outcome after treatment of pulmonary atresia with ventricular septal defect: nationwide study of 109 patients born in 1970–2007. Eur J Cardio-Thorac Surg 2016; 49 1411–1418.
2. Balaguru D, Dilawar M. Pulmonary atresia with ventricular septal defect: Systematic review. Heart Views 2007; 8(2): 52-61.
3. Kilner PJ. The Role of Cardiovascular Magnetic Resonance in adults with congenital heart disease. Prog Cardiovasc Dis. 2011; 54(3): 295–304.
4. Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, Eichhorn J, Sarikouch S, Greil GF, Beerbaum P, Bucciarelli-Ducci C, Bonello B, Sieverding L, Schwitter J, Helbing WA. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. EHJ–CVI 2015; 16: 281–297.
5. Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. JCMR 2013; 15:51.
6. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. JCMR 2011; 13:9.
7. Rushani D, Kaufman JS, Ionescu-Ittu R, Mackie AS, Pilote L, Therrien J, Marelli AJ. Infective endocarditis in children with congenital heart disease cumulative incidence and predictors. Circulation. 2013; 128:1412-1419.
8. Ugaki S, Rutledge J, Al Aklabi M, Ross DB, Adatia I, Rebeyka IM. An increased incidence of conduit endocarditis in patients receiving bovine jugular vein grafts compared to cryopreserved homograft for right ventricular outflow reconstruction. Ann Thorac Surg 2015; 99:140–7.
Case prepared by:
Jason Johnson, MD MHS
Associate Editor, SCMR Case of the Week
Le Bonheur Children’s Hospital
University of Tennessee Health Science Center





