Guillem Pons-Lladó, MD, PhD and Ester Bertolí-Inglés, Nurse & CMR Technologist.
Cardiac MR Unit, Department of Diagnostic Imaging, Clínica Corachán, Barcelona, Spain.
Clinical history
The case of a 85 year-old lady is presented who complain of atypical chest pain. After a markedly abnormal ECG showing high voltage R waves and deep, inverted T waves on precordial leads, an echocardiographic study is performed, suggesting non-obstructive hypertrophic cardiomyopathy (HCM).
A CMR exam is requested to: 1) confirm the diagnosis; 2) precisely determine the extent of the process; 3) establish the presence or not of myocardial fibrosis; and 4) to rule out myocardial ischemia.
CMR findings
Long-axis cine sequences (Video 1) show asymmetrical left ventricular hypertrophy located at a distal segment of the left ventricular chamber, with preserved contractile function and systolic obliteration of the cavity. Also, an area of wall thinning and akinesia is seen at the apical region (Figure 1, arrows).
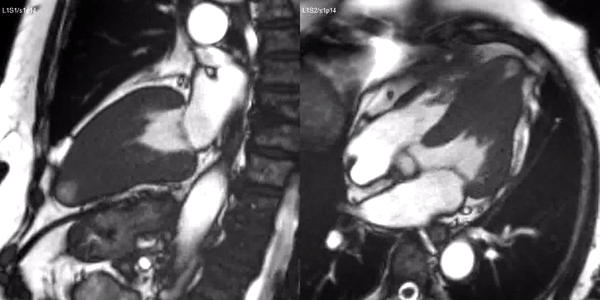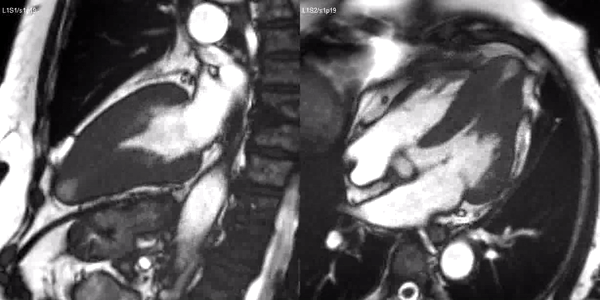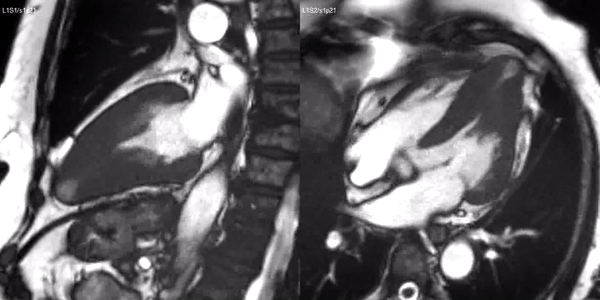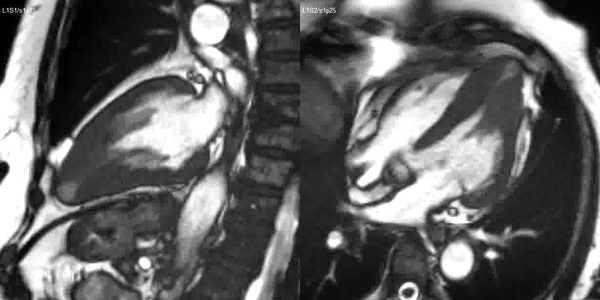
Video 1. SSFP 2 and 5-chamber demonstrating apical hypertrophy with thinning and akinesia at the apical region.
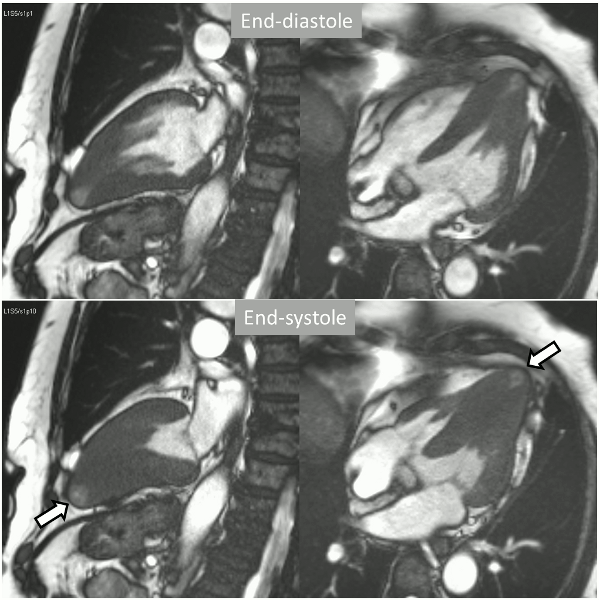
Figure 1. SSFP 2 and 5-chamber in end-diastole and end-systole demonstrating apical hypertrophy and apical aneurysm.
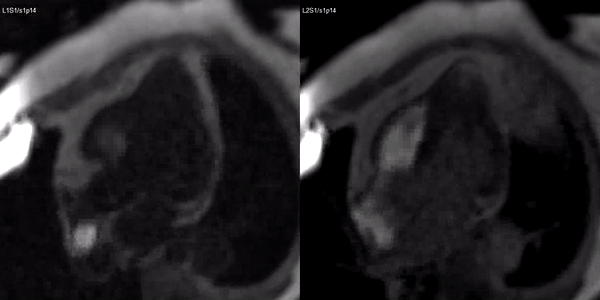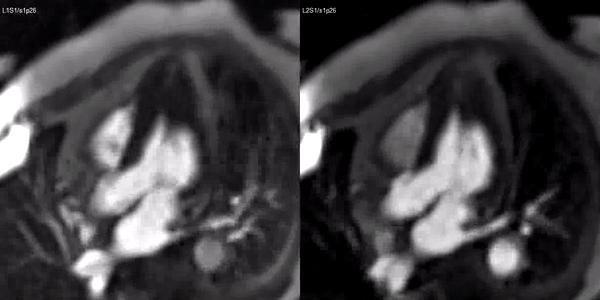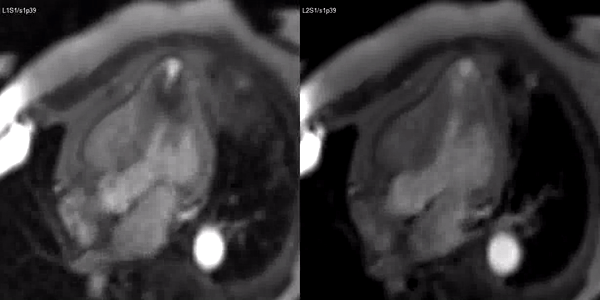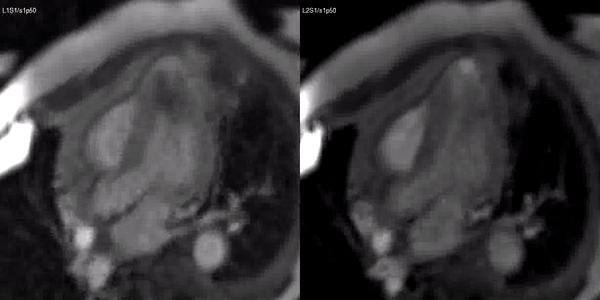
A stress adenosine and rest myocardial perfusion study was performed on simultaneous short-axis and 4-chamber planes (Videos 2 and 3), where a circumferential, transmural, reversible defect is detected at the level of the hypertrophied segment (Figure 2, arrows).
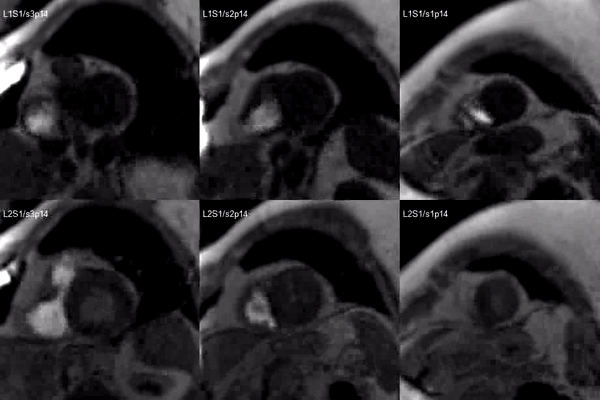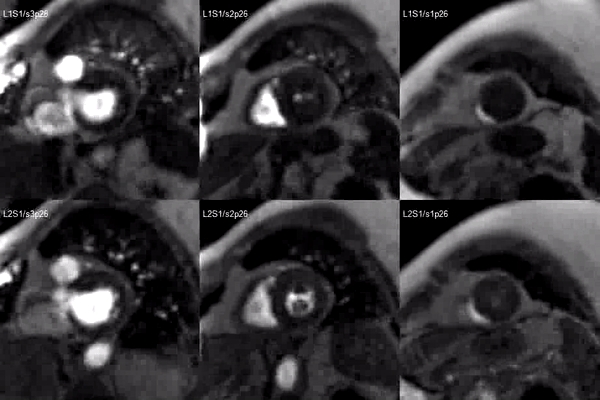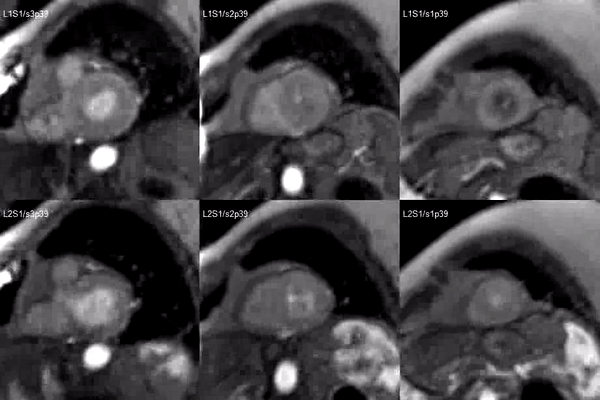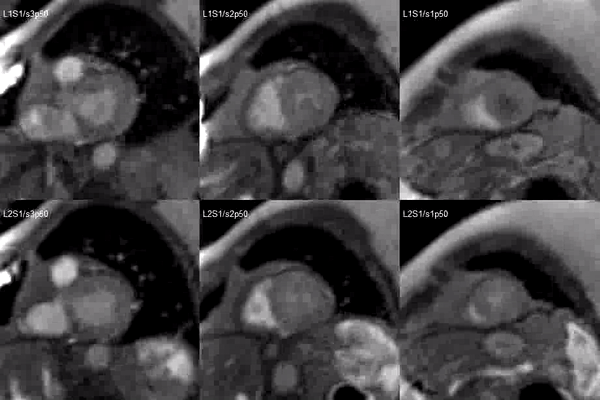
Video 2. Short axis base (left), mid (middle) and apex (right) stress (top) and rest (bottom) first pass perfusion mri demonstrating circumferential subendocardial inducible ischemia at the apical segments.
Video 3. Stress (left) and rest first pass perfusion mri in the 3-chamber view demonstrating circumferential subendocardial inducible ischemia at the apical segments.
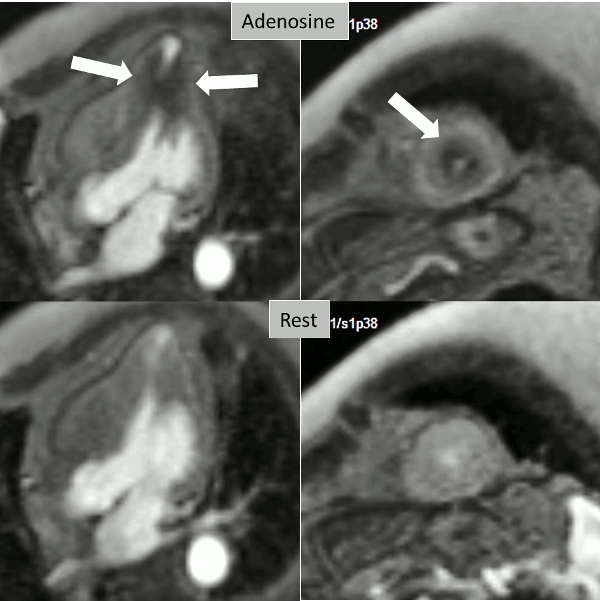
Figure 2. Stress (top) and rest (bottom) first pass perfusion mri demonstrating circumferential subendocardial inducible ischemia (arrows) at the apical segments.
Finally, delayed contrast enhancement was detected at Inversion-Recovery sequences involving the thin apical region (Figure 3, arrows).
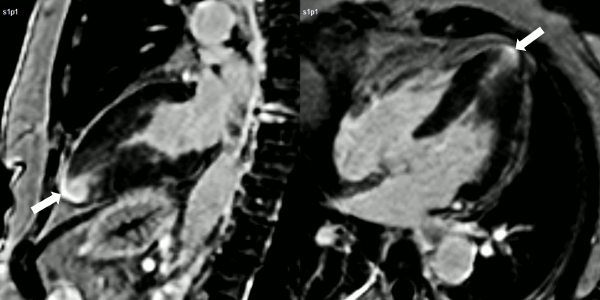
Figure 3. Late gadolinium enhancement demonstrating apical myocardial delayed enhancement.
Conclusion
The diagnosis of HCM is confirmed, with asymmetrical hypertrophy located at a distal level which, given the hypercontractile state of the left ventricle, leads to systolic obliteration of the cavity. The apical tip of the chamber shows wall thinning and protruding shape at both end-diastole and end-systole, together with transmural delayed contrast enhancement, indicating myocardial necrosis of this segment and aneurysm formation. There is no other evidence of intra-myocardial fibrosis. Interestingly, the first-pass study shows markedly abnormal perfusion defect under adenosine that precisely involves the hypertrophied segment, and is not present at rest.
After the results of the CMR study showing inducible perfusion defect, a coronary angiography was performed demonstrating tortuous vessels without significant obstructive lesions.
Perspective
The association of asymmetrical mid-ventricular or mainly apical hypertrophy with aneurysm formation of the very apical tip of the chamber, in the absence of epicardial coronary atherosclerotic lesions, was early recognized as a part of the spectrum of HCM (1). Known to occur in 2% of patients with HCM when large series have been studied (2), this association has shown to portend a particularly adverse prognosis (3).
Although no definite mechanism has been proven for the development of apical myocardial scarring and aneurysm formation in the presence of mid-ventricular or distal hypertrophy, an ischemic origin seems plausible for this association, despite of normal epicardial coronary arteries. The presence of mid-ventricular hypertrophy and systolic obliteration would lead to pressure overload of the apical region with increased oxygen demand (4). This, together with the abnormalities of small vessels (i.e.: reduced capillary density, medial layer hyperplasia, perivascular fibrosis) known to occur in HCM (5) would lead to necrosis, progressive dilatation, and aneurysm formation in the apical region.
Defective myocardial perfusion in HCM has been shown by CMR stress adenosine studies and attributed to microvascular dysfunction (6). In mid-ventricular obstruction with apical aneurysm, however, and despite of an early study showing perfusion defects at the hypertrophied region by radionuclide (7), CMR observations have not been reported up to date (8).
The presence, in this case, of a sharp inducible perfusion defect at the site of hypertrophy clearly supports the mechanism discussed above. The information of the CMR study provides relevant information for the management of this particular form of HCM, which, once known, should include arrhythmia investigation, potential prophylactic anticoagulant treatment and, particularly, a close surveillance of the patient (9), provided that the presence of inducible ischemia may result, according with the mentioned pathophysiological process, in further extension of the scarred myocardial region.
References
5. Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology 2004; 44:412–427.
Link to full case images: https://www.cloudcmr.com/2762-1002-5148-0648/
Case prepared by Eddie Hulten, MD, Associate Editor, SCMR Case of the Week





