Case from: Heiko Kindler, Rick Wage, Gerard King, John Clarke, Stuart Cook
Institutes: Eagle Lodge Cardiology, Limerick, Ireland and Blackrock Clinic, Dublin, Ireland. Duke-NUS, Singapore
Clinical history: We describe a case of a teenager (Sibling A) who collapsed during athletic activity, has T wave inversion on ECG. He made a full recovery and no cardiac arrhythmia was identified on Holter monitoring. The event lead to family screening of a sibling (Sibling B, female, 20s) and the parents. There are two family members with HCM, one maternal granduncle a maternal grandaunt (Figure 1). The index case (sibling A) was found to have widespread T wave inversion on ECG (Figure 2).
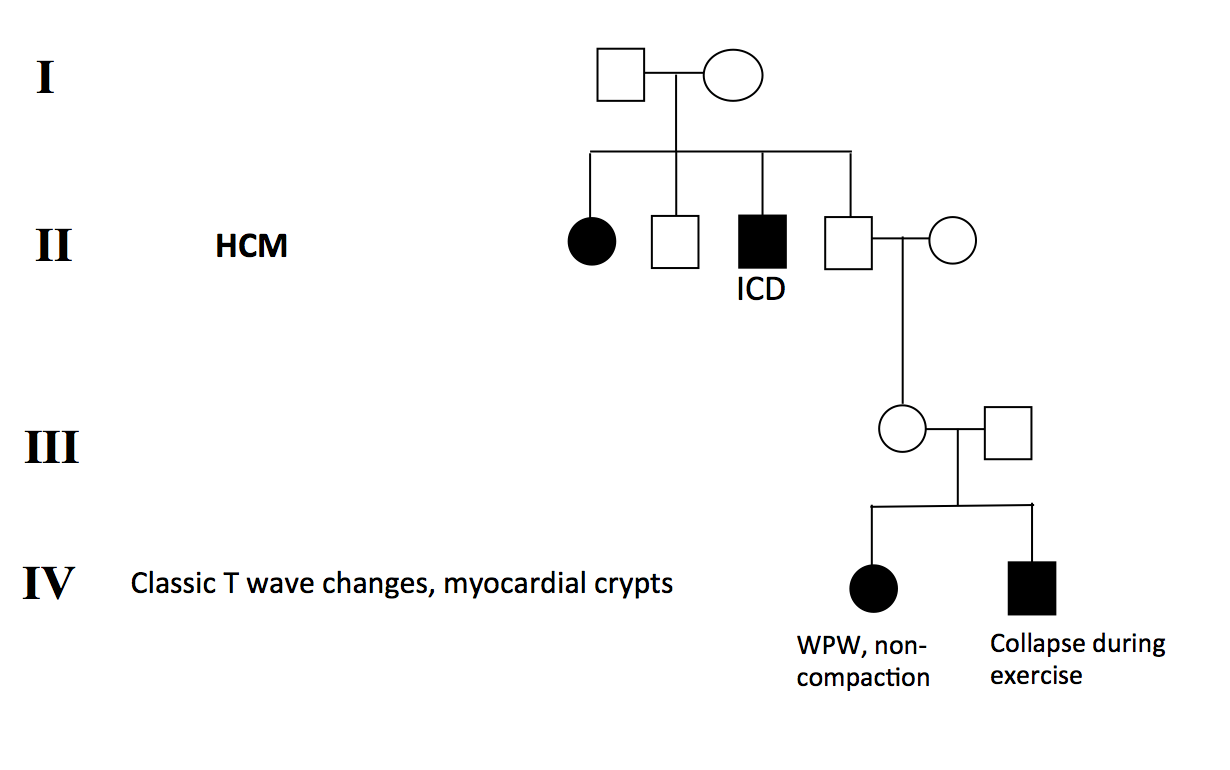
Figure 1: Pedigree analysis of the family
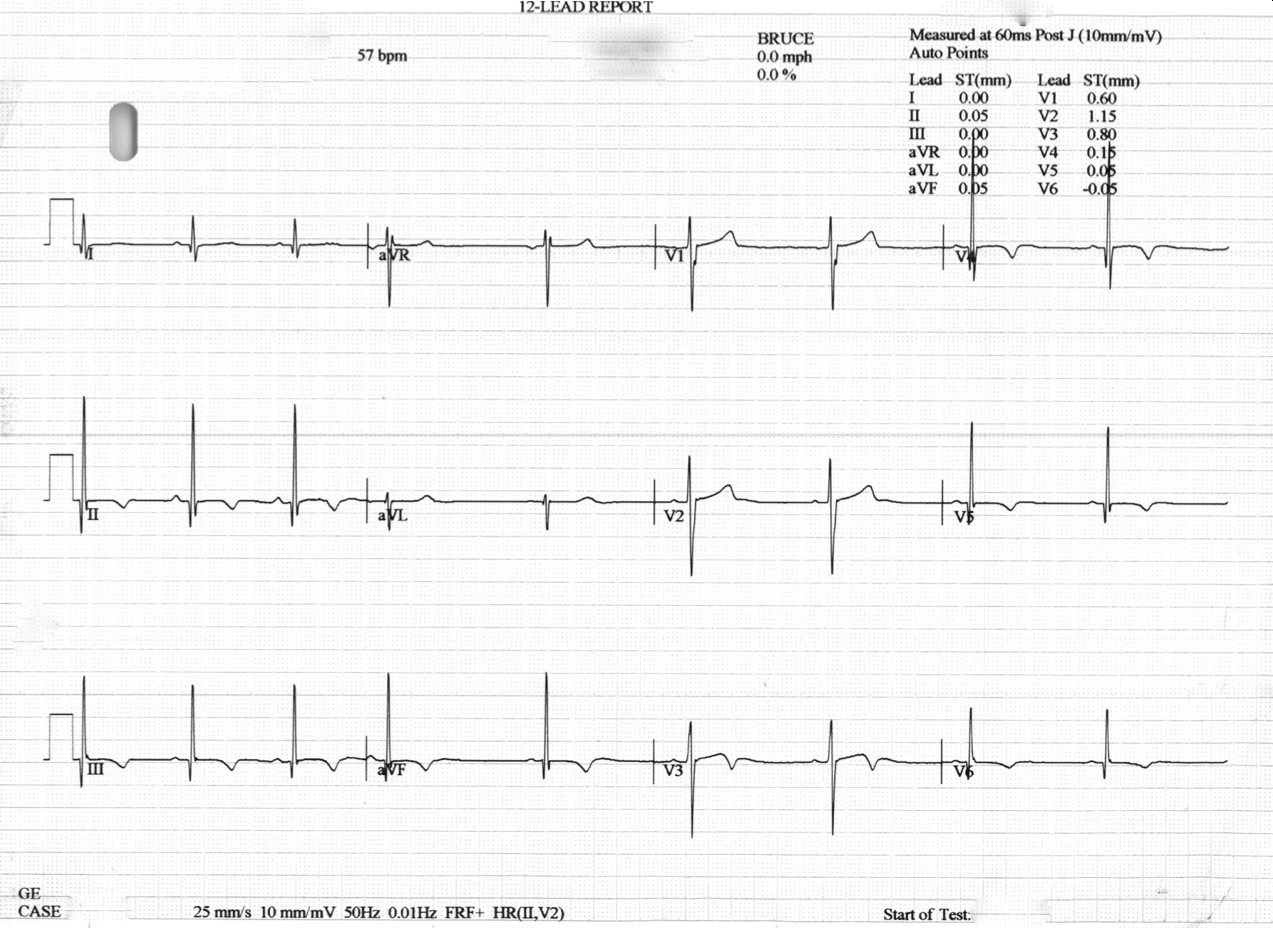
Figure 2: Index patient ECG, indicating LVH and demonstrating widespread T-wave inversion.
CMR Findings: CMR revealed deep myocardial crypts without hypertrophy and frond-like trabeculations (Figure 3 and Movie 1).
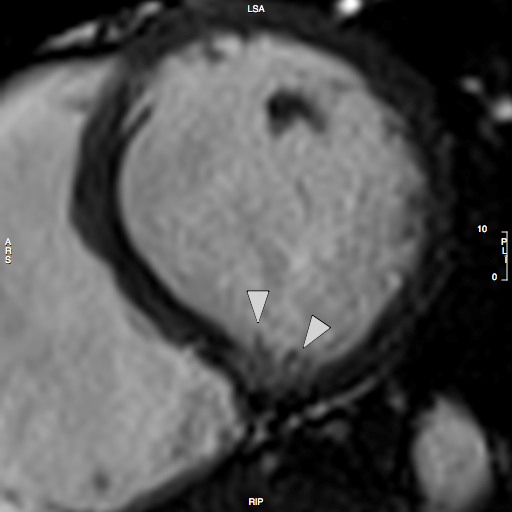
Figure 3: Short-axis CINE still demonstrating Crypts and frond-like trabeculations.
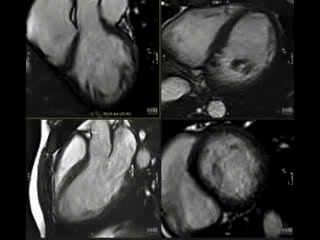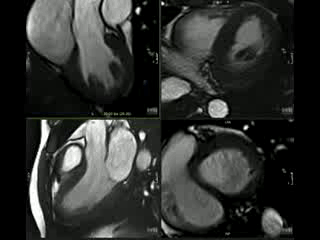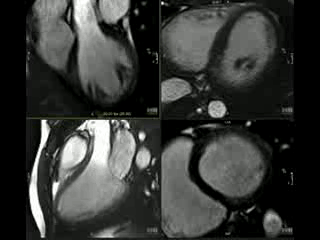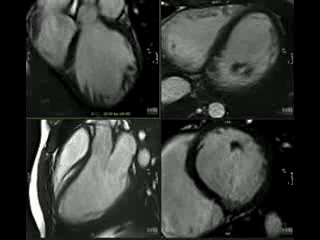
Movie 1: Short-axis and LVOT CINE demonstrating Crypts and frond-like trabeculations.
Sibling B Has Wolff Parkinson White Syndrome, widespread T wave inversion, a long QTc interval, 490ms by Bazett’s eq. (Figure 4) as well as marked apical myocardial trabeculation with preserved LV ejection fraction (Movie 2). Myocardial crypts are demonstrated at the inferior wall of the vertical long axis slice, however, they are not easily appreciated on the short axis slice because the short axis slice transsects the myocardium at a level just between two crypts (Figure 5).
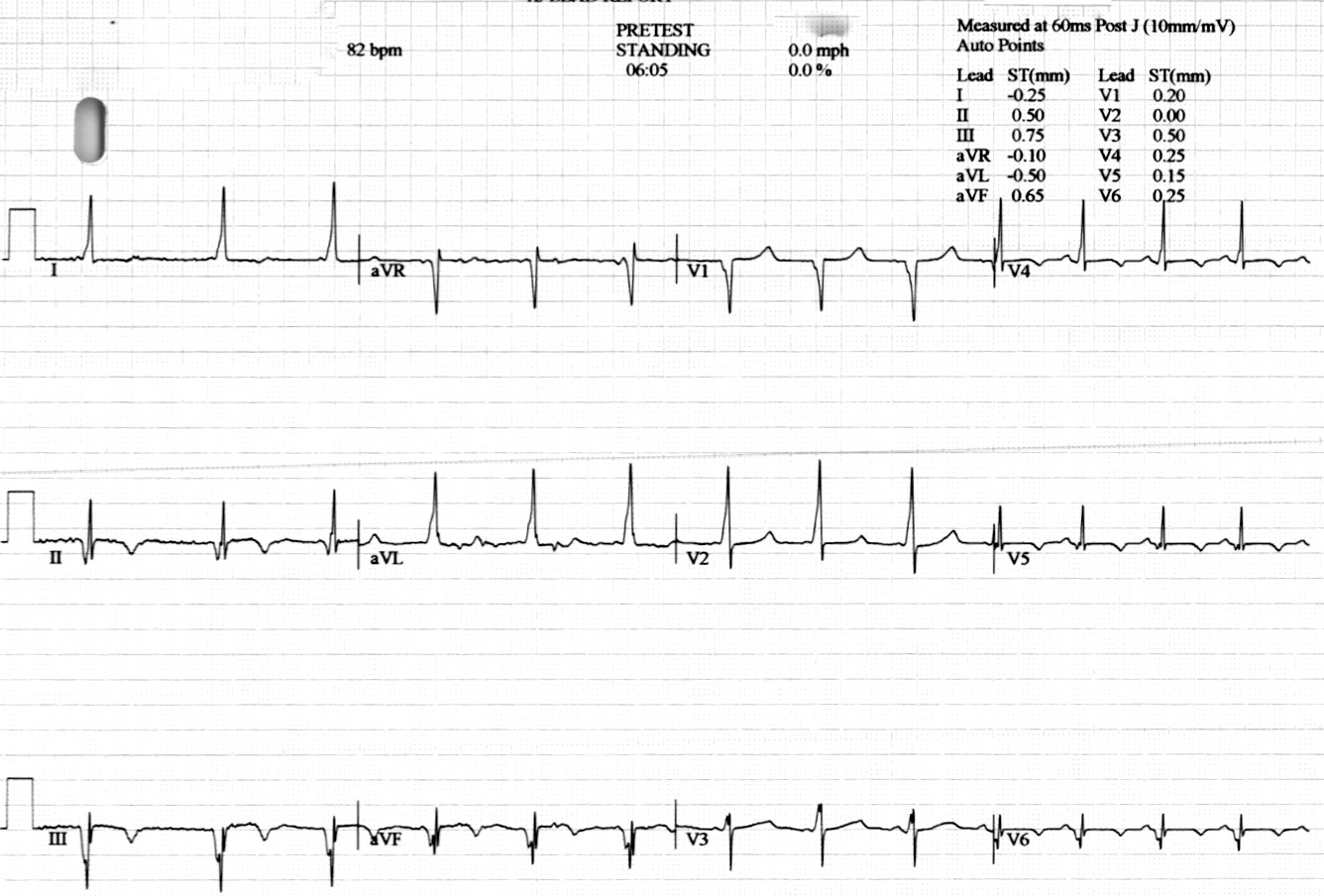
Figure 4: 12-lead ECG of sibling B demonstrating short PR interval, delta-waves and T wave abnormalities.
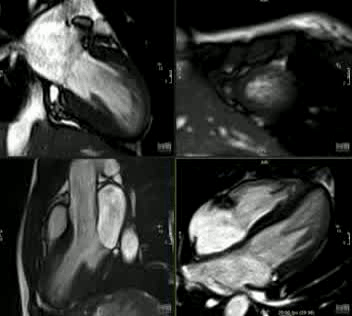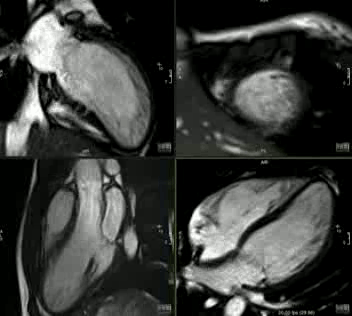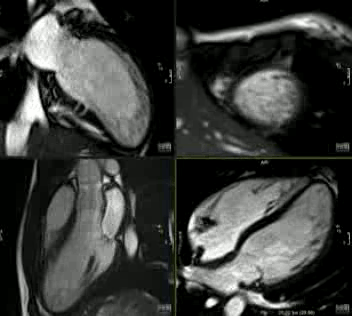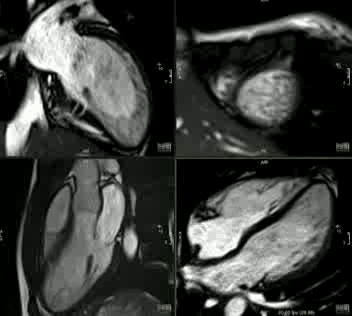
Movie 2: CINE imaging depicts marked apical myocardial trabeculation with preserved LV ejection fraction.
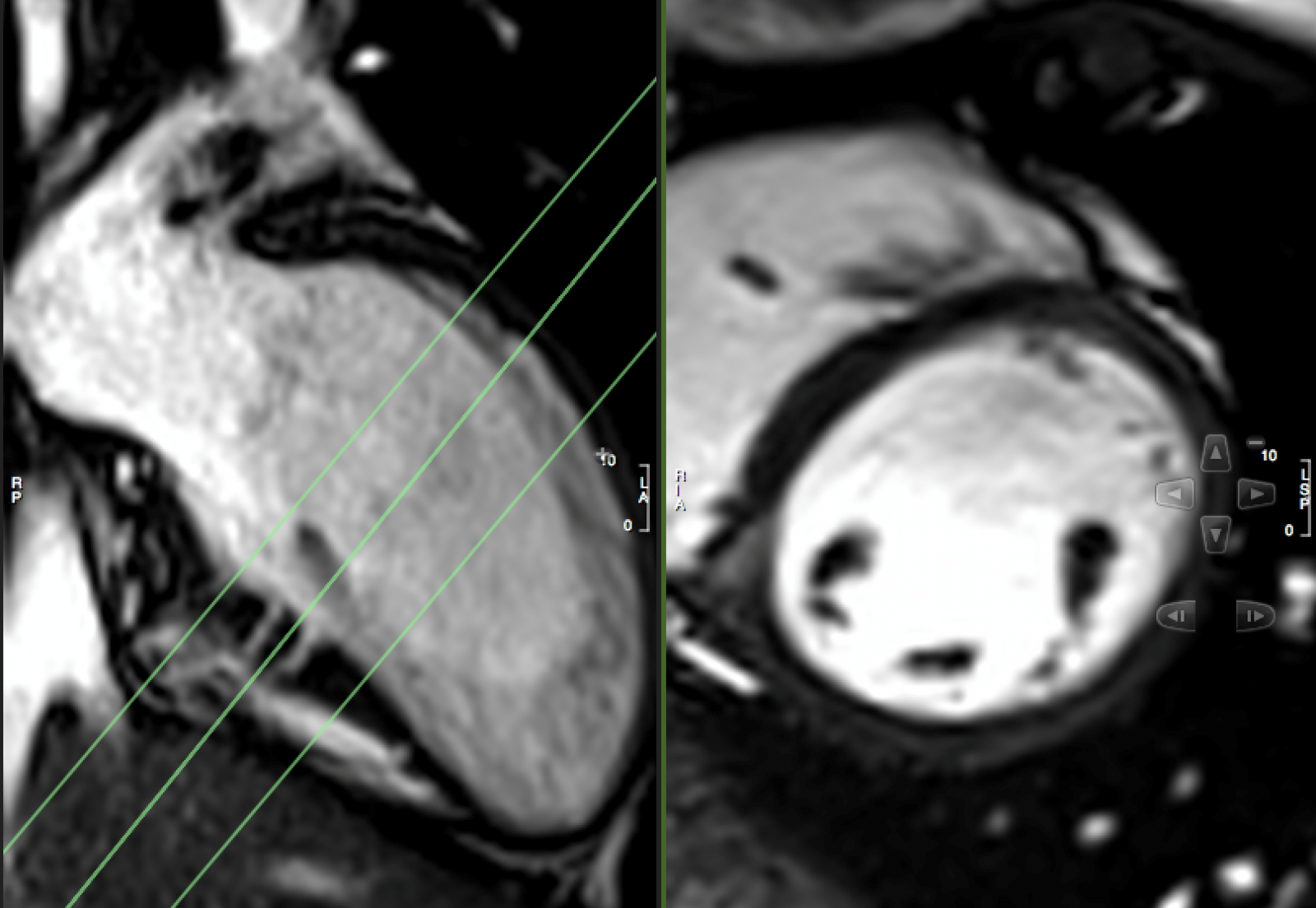
Figure 5: VLA and SAX CINE still.
The private history is not available and since the relationship between the proband and the family members with definite hcm is not that close, there could be two diseases running in the family.
Myocardial Crypts
There is controversy in the recent literature about the clinical relationship between Myocardial Crypts and Hypertrophic Cardiomyopathy (HCM). Myocardial Crypts have been reported to be a specific feature of pre- hypertrophic mutation carriers who do not have overt signs of the disease on Cardiac MRI. [1, 2]. Other authors have found that these anatomical anomalies also occur in healthy volunteers as well as other conditions such as pulmonary stenosis [3, 4]. Myocardial crypts have also been reported in over 60% of 31 genotype-positive/phenotype-negative relatives of patients with HCM who have no evidence of LV hypertrophy [5]. Our observation lends further support to the argument that there may be genetic relationship between HCM and Myocardial Crypts.
This is the first description of myocardial crypts in kindreds with widespread T wave inversion with left ventricular hypertrophy and several deep myocardial crypts. One sibling is has features of marked left ventricular apical trabeculation with normal LV ejection fraction (Figure 5 and Movie 2).
Myocardial Crypts are narrow, blood-filled invaginations within the LV wall that can take various shapes and sizes. They have been reported to occur in various conditions. These myocardial crypts or cavities, are generally more easily appreciated by cardiovascular magnetic resonance imaging than by echocardiography. The occurence of these crypts have been reported by Maron et al in 61% of 31 phenotype-negative but genotype-positive patients with hypertrophic cardiomyopathy. Myocardial crypts have also been described in patients after surgical repair of congenital pulmonary stenosis as well as healthy volunteers [3]. Furthermore, a link has been described between HCM, noncompaction cardiomyopathy and Wolff-Parkinson-White syndrome [7].
There is considerable genetic heterogeneity, with an overlap of different phenotypes, and the variability of hereditary patterns, raise the questions whether there is a morphological trait from dilated/hypertrophic cardiomyopathy to LVNC and what are the triggers and modifiers to develop either dilated, hypertrophic cardiomyopathy, or LVNC in patients with the same mutation and there seems to be a shared molecular aetiology of different cardiomyopathic phenotypes, including LVNC, hypertrophic and dilated cardiomyopathies [6].
The difficulty of imaging of myocardial crypts: You may only find what you are looking for
Previous studies assessing the frequency and characteristics of myocardial crypts have been retrospective and myocardial crypts are not easily appreciated in all studies, in particular their significance being contentious. MC may be “under-scanned” and “under-reported”.
It is important for both radiographers as well as reporting Cardiologist/Radiologist to be aware of the potential clinical implications of these crypts, in particular when there is a family history or other genetic pointers. clearly identify myocardial crypts as the slice may transsect the myocardium at a level just between two crypts (Figure 5). In addition, patient movement, arrhythmia and through plane motion may have a degrading effect on image quality.
Conclusion and perspective: Whether the above described anatomical anomalies are in every case related to HCM or whether they are no more than incidental variants of local myocardial structure [3] is a matter of ongoing debate. It is important to be cautious in the interpretation of the clinical significance of new findings and their prognostic value. Myocardial crypts in siblings with similarly abnormal resting ECGs and a family history of HCM, described in this particular case, adds to the mounting evidence that there may be a genetic link between myocardial crypts and HCM in some cases. We speculate that Myocardial Crypts may either represent a forme fruste of HCM in some of these cases, similarly to those that have been described in Marfan Syndrome, or an early or mild pre-clinical form of HCM where only mild features of the syndrome become apparent.
Current studies may have underreported these crypts owing to their coincidental nature and contentious etiology. Larger scale prospective international registry studies may be needed and scanning protocols may need to be adapted to reflect the location and elusive nature of these myocardial structural anomalies.
References:
1. Germans T, Wilde AA, Dijkmans PA, et al. Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. Journal of the American College of Cardiology 2006;48(12):2518-23
2. Brouwer WP, Germans T, Head MC, et al. Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers. European heart journal cardiovascular Imaging 2012;13(4):292-7.
3. Petryka J, Baksi AJ, Prasad SK, et al. Prevalence of inferobasal myocardial crypts among patients referred for cardiovascular magnetic resonance. Circulation Cardiovascular imaging 2014;7(2):259-64.
4. Puntmann VO, Jansen C, Nagel E. Letter by Puntmann et al regarding article, “Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy”. Circulation Cardiovascular imaging 2012;5(5):e66; author reply e67
5. Maron MS, Rowin EJ, Lin D, et al. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circulation Cardiovascular imaging 2012;5(4):441-7
6. Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? European heart journal 2011;32(12):1446-56
7. Alday, L., Moreyra, E., Bruno, E., Rossi, N., & Maisuls, H. (2010). Left ventricular noncompaction associated with hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome. Health (1949-4998), 2(3).
COTW handling editor: Pranav Bhagirath, MD





