Case from: Karima Addetia, MD, Elizabeth Retzer, MD, Roberto M. Lang, MD, Amit R. Patel, MD.
Institute: University of Chicago, Chicago, IL, USA.
Clinical history:
A 34 year-old female with a 2-month history of shortness of breath and new onset lower extremity edema presented to the emergency department for assessment. Two months prior to the presentation she was bedridden for 3 days with flu. She had no past medical history and was not taking any medications. She is a smoker (1/2 pack per day) and drinks ~42 shots of liquor per week. Her initial examination was consistent with congestive heart failure. Preliminary lab work revealed a Troponin-T of 0.40 ng/mL and NT –proBNP levels of 9386 pg/mL.
Chest x-ray (Figure 1) and EKG (Figure 2) are shown
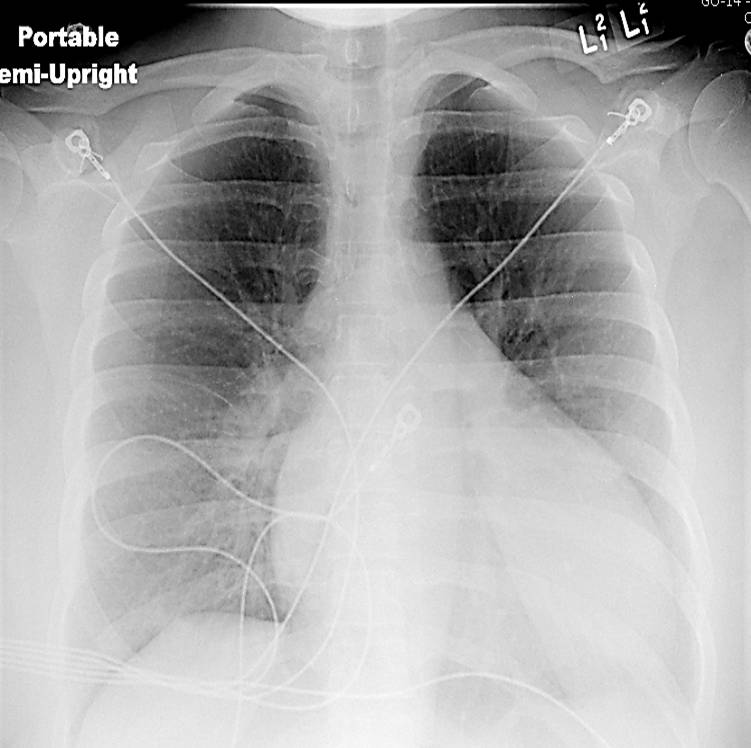
Figure 1. Chest x-ray showing an enlarged cardiac silhouette
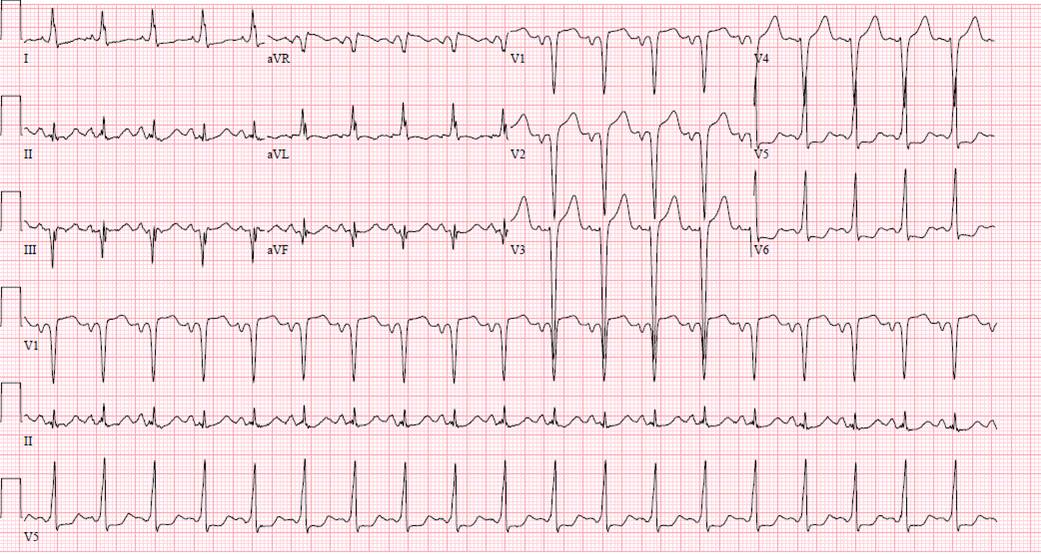
Figure 2. EKG showing sinus tachycardia, q waves in the high anterior leads and ST-T wave changes in the lateral leads which could be suggestive of ischemia
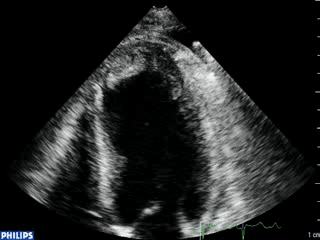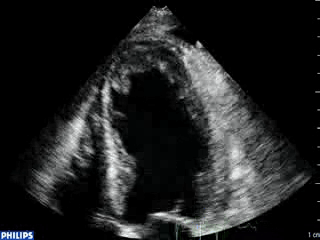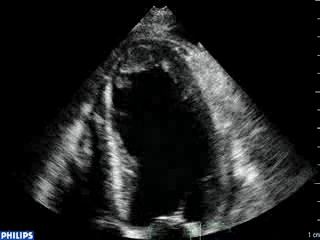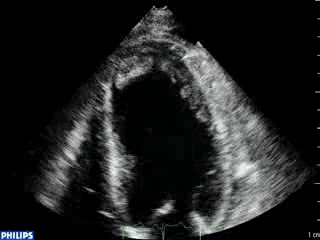
An echocardiogram was ordered (Movie 3) and revealed concentric left ventricular (LV) hypertrophy, LV ejection fraction 17% and severe global hypokinesis with preserved basal segments. There was a thrombus at the LV apex and moderate mitral regurgitation. A cardiac MRI (Movie 4) with late gadolinium enhancement (LGE) imaging (Figure 5) was ordered as further work-up.
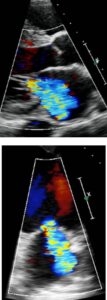
Color Doppler recording in the parasternal long axis (top) and apical two-chamber (bottom) showing moderate mitral regurgitation.
CMR Findings:
Steady state free precession (SSFP) cine images (Movie 4) revealed severe akinesis of inferior, inferoseptal, anterior, and lateral walls with preserved function of the LV basal segments. The apex was dyskinetic with thrombus. LGE clearly outlined the thrombus at the LV apex (Figure 5). In addition, there was extensive subendocardial, non-transmural LGE involving the entire inferior, inferolateral, mid-apical and anterolateral walls as well as the apical septum in a pattern consistent with myocardial infarction due to multivessel coronary disease (Figure 5). The majority of the territory appeared viable.
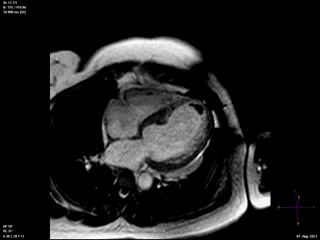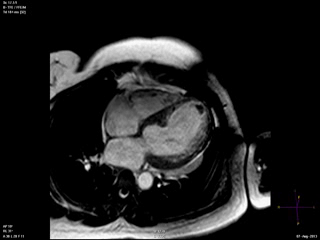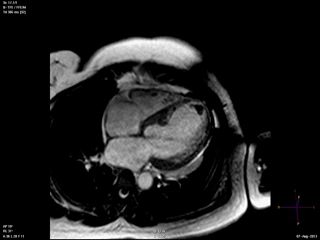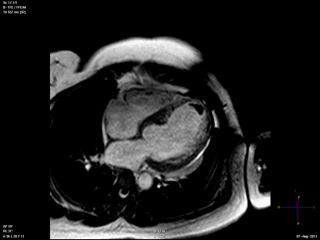
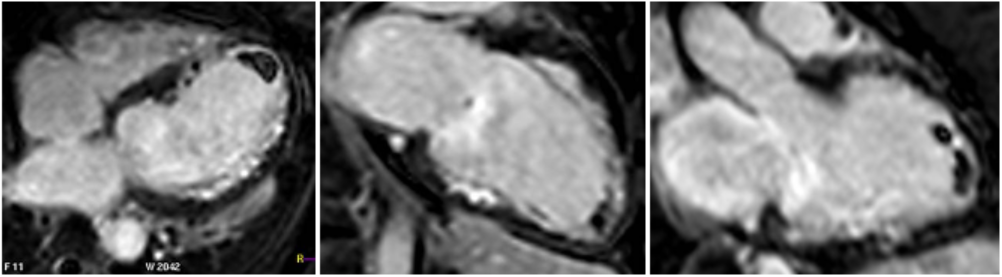
Figure 5. LGE images showing extensive subendocardial, non-transmural LGE involving the entire inferior, inferolateral, mid-apical and anterolateral walls as well as the apical septum
Conclusion:
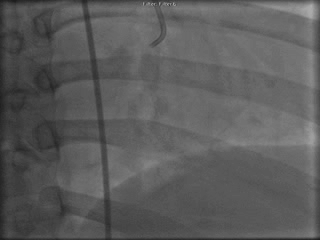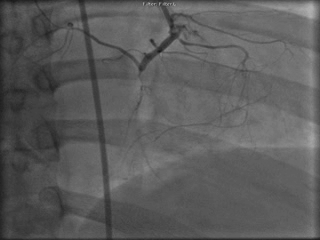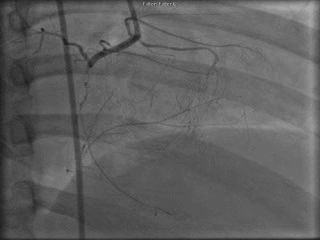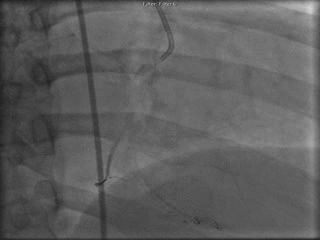
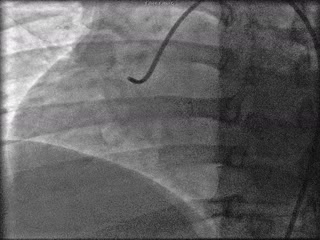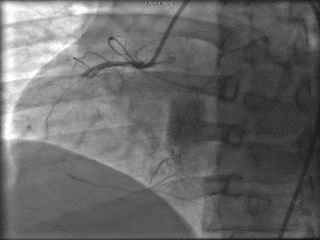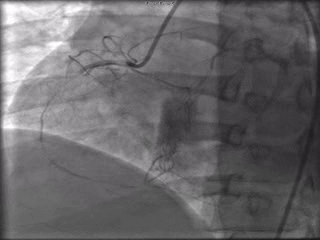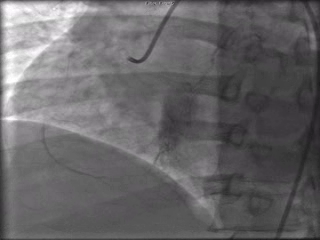
The patient was taken to the cardiac catheterization laboratory and was found to have a severely stenotic lesion in the midportion of the left anterior descending artery which was dilated and stented with overlapping drug-eluting stents. The circumflex had only mild diffuse luminal irregularities. The right coronary artery had a chronic total occlusion at its proximal segment and filled via left-to-right collaterals.
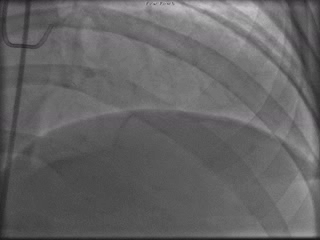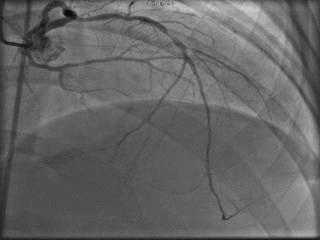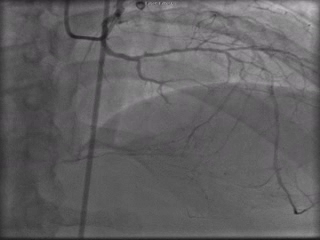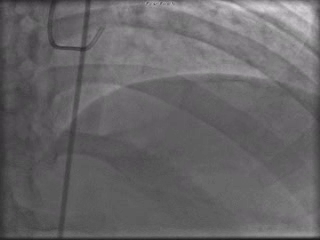
Perspective:
It has been documented in the literature that an end-diastolic wall thickness of <5-6 mm (measured on CMR or echocardiography)1,2 suggests irreversible myocardial damage. In our case, the apex is thinned and the septal apex measured <5 mm on both echocardiography and CMR (Figure 6) suggesting that this area might not be viable.
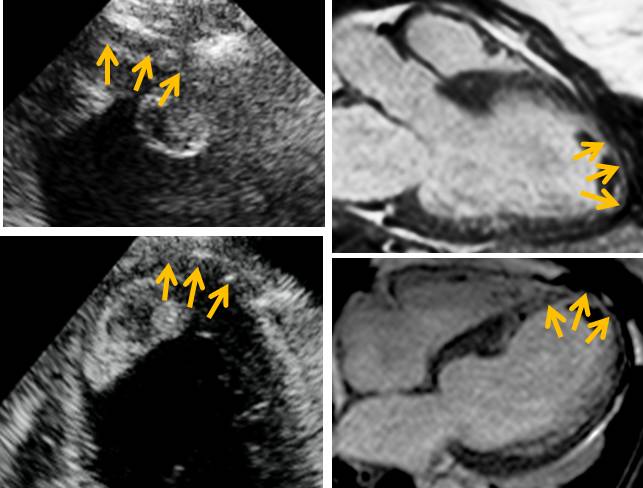
Figure 6. Most of the apex and especially the septal apex (inferior and anterior) are thinned (orange arrows, end-diastolic measurement < 5 mm). This is true on echocardiography (top left, apical 3-chamber view with focus on the apex and bottom left, apical 4-chamber view with focus on the apex) and CMR (top right 3-chamber axial view and bottom right, 4-chamber axial view).
A recent study showed, in a group of 201 patients, with wall thinning (defined as end-diastolic wall thickness ≤5.5 mm), that patients with limited or no myocardial scar, as defined by LGE-CMR, had contractile improvement following revascularization. Limited scar was defined as a scar area on short axis LGE images comprising less than or equal to 50% of the total area of myocardium subtended by the scar. We calculated the regional extent of scar for our patient (Figure 7) and found that while the septal apex and distal inferoseptum appeared non-viable (Figure 8) all the other segments with LGE were viable.
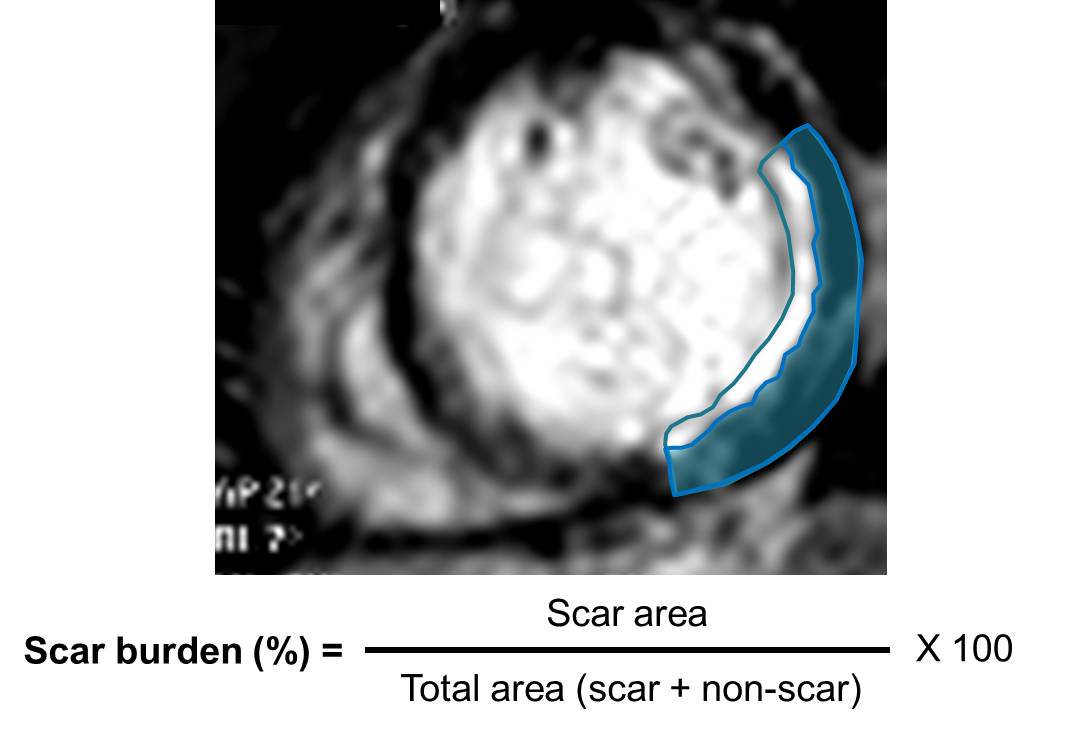
Figure 7. Sample calulation of scar burden for our patient. At this level the scar burden would be estimated at 60%
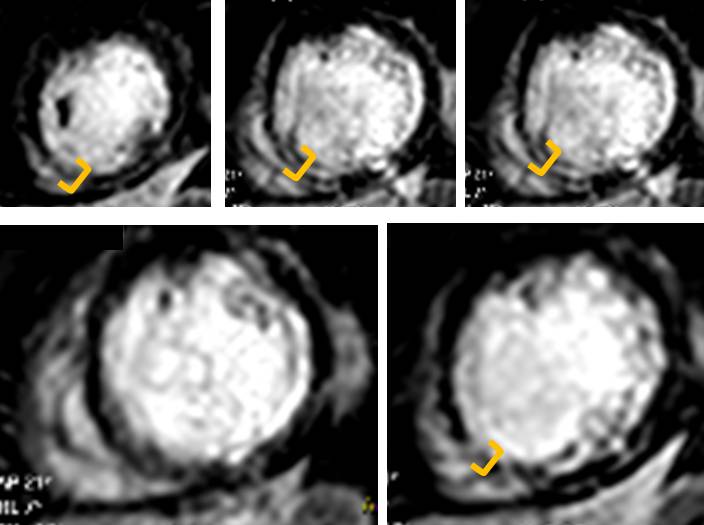
Figure 8. Septal apex and proximal inferoseptum are non-viable. Note transmural LGE top and bottom right (in between orange brackets)
Taking our results together with the results of the this study, there is a good chance that at least the portion of the myocardium supplied by the LAD should recover (and possibly also the portion supplied by the RCA as well since most of the RCA territory depended completely on collateral circulation). The likelihood of myocardial recovery after revascularization decreases progressively with increased thickness of myocardial scar4,5.
In summary, in this case, documentation of LGE in a pattern consistent with myocardial infarction (i.e. subendocaridal distribution in the territories associated with one or more coronary arteries) helped to rule out myocarditis and alcohol abuse as possible etiologies for this patient’s cardiomyopathy. Furthermore, when considering the quesion of myocardial recovery, thinning of the myocardium alone does not provide information about viability. LGE CMR helps to assess this finding more carefully. Of note, in the above-mentioned study, patients with myocardial thinning and limited scarring tended to have significant stenosis (>95%) in the vessel subtending the region3. Our patient was no exception.
References:
1. Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, Erdmann E. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048.
2. Cwajg JM, Cwajg E, Nagueh SF, He ZX, Qureshi U, Olmos LI, Quinones MA, Verani MS, Winters WL, Zoghbi WA. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to rest-redistribution T1-201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol. 2000;35:1152-1161.
3. Shah DJ, Kim HW, James O, Parker M, Wu E, Bonow RO, Judd RM, Kim RJ. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309:909-918.
4. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
5. Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535-1541
COTW handling editor: Karima Addetia, MD





