Case from: Suzanne de Waha, Ingo Eitel, Steffen Desch, Philipp Lurz, Georg Fuernau, Gerhard Schuler, Holger Thiele.
Institute: University of Leipzig – Heart Center, Department of Internal Medicine – Cardiology, Leipzig, Germany.
Clinical history: A 43-year old man with unremarkable past medical history presented with progressive dyspnea and peripheral edema. ECG showed sinus bradycardia, right bundle brunch block and frequent polymorphic ventricular ectopic beats (Figure 1).
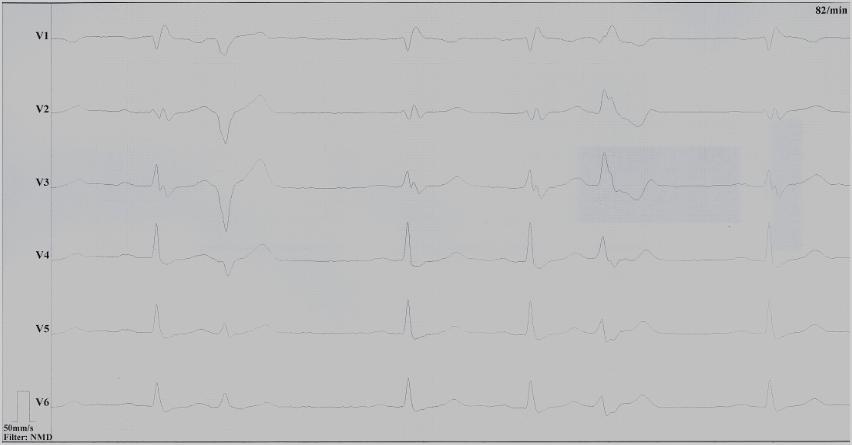
Figure 1: ECG showing sinus bradycardia, right bundle brunch block and polymorphic ventricular ectopies. (click to enlarge)
Blood tests including troponin and inflammatory markers were within normal limits. Echocardiography revealed an impaired left ventricular (LV) and right ventricular (RV) function with normal LV and RV dimensions and regional wall motion abnormalites in the basal septum and lateral wall (Movie 1).
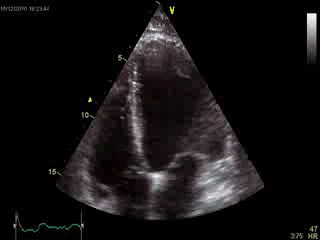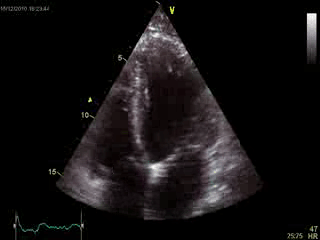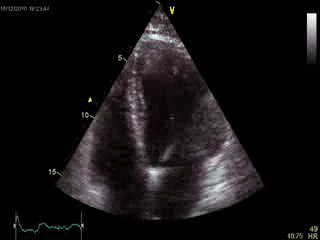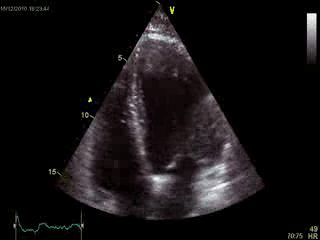
Movie 1. Transthoracic echocardiography 4-chamber view demonstrating globally reduced LV and RV function with normal LV and RV dimension and regional wall motion abnormalities.
Coronary angiogram did not show coronary artery disease. Cardiac magnetic resonance (CMR) imaging was requested to further investigate the cause of LV dysfunction.
CMR Findings:
Cine imaging confirmed impaired LV and RV ejection fraction (LV-EF 38%, RV-EF 30%) with hypokinesia in the LV basal inferior and basal to midventricular lateral segments, as well as akinesia of the basal septum (Movie 2)
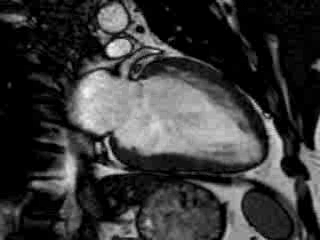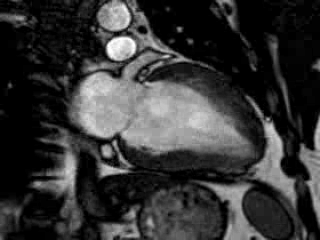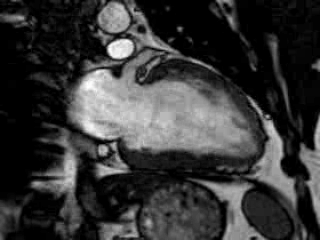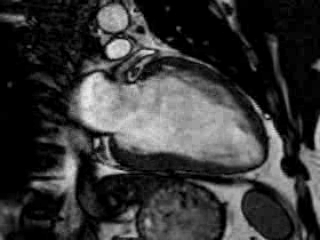
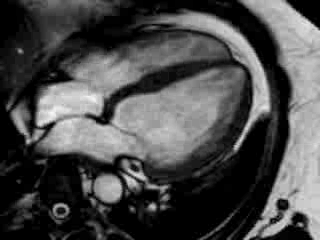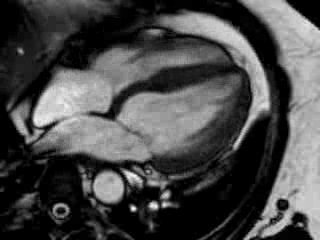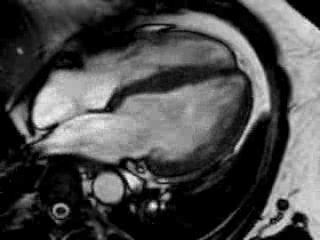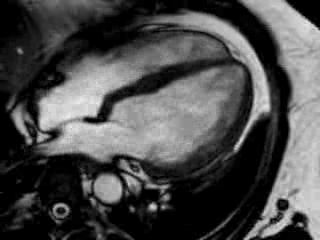
Movie 2 Baseline cine 2-chamber view (left) and 4-chamber view (right) showing reduced LV and RV function with regional wall motion abnormalities.
T2-weighted imaging did not show areas of increased signal intensity indicative of edema. Late-gadolinium enhancement imaging (Figure 2) revealed extensive patchy fibrosis matching regional wall motion abnormalities: hyperenhancement was found in the RV wall, the basal septum, as well as in the mid LV anterolateral and inferolateral wall, with a predominantly subepicardial pattern.
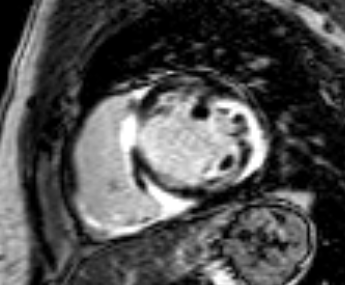
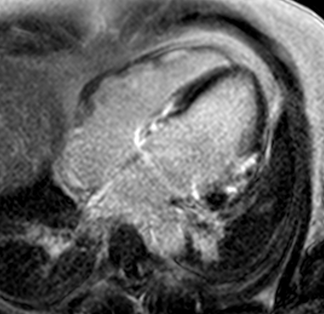
Figure 2. Late-enhancement imaging at baseline in short axis (left panel) and 4-chamber view (right panel) showing extensive fibrosis matching regional wall motion abnormalities.
Management and Clinical Course: Endomyocardial LV biopsies revealed slightly increased myocardial fibrosis without any sign of acute, chronic or granulomatous myocarditis (Figure 3a and 3b). Computed tomography demonstrated non-specific pulmonary infiltration and mediastinal lymph nodes of borderline size (Figure 4). Biopsy of these lymph nodes was not performed due to the risk related to the procedure and uncertain additional diagnostic benefit.
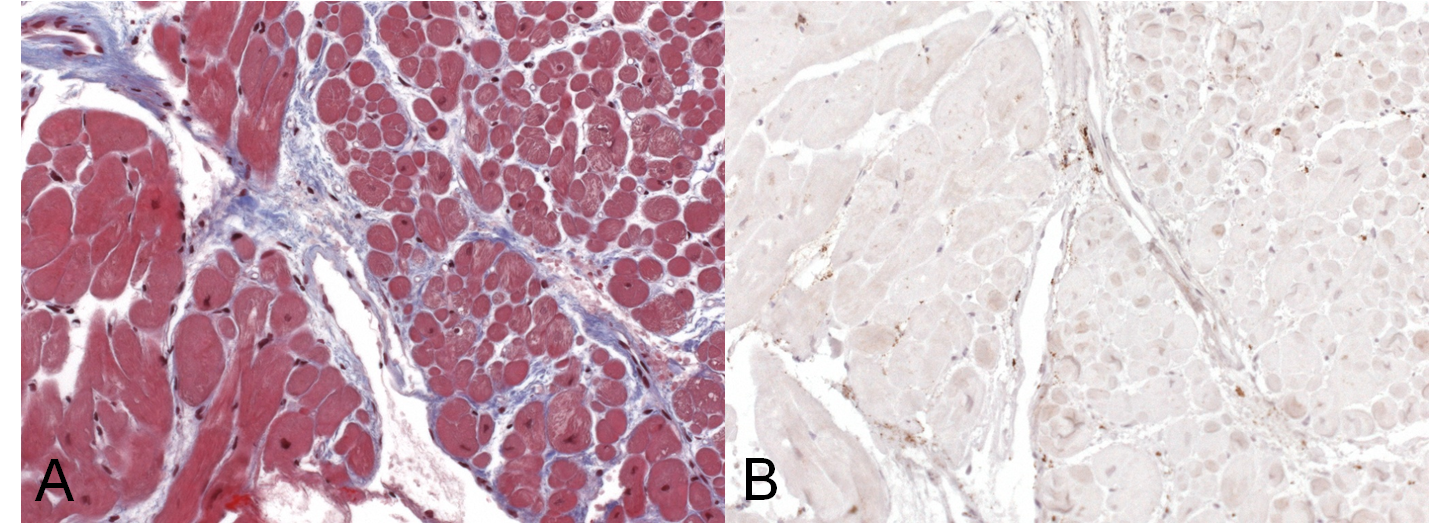
Figure 3 a) Trichrome staining of endomyocardial biopsies detecting zones of diffuse myocardial fibrosis (blue). b) Immunohistology of endomyocardial biopsies displaying no evidence of infiltration of CD3 T-lymphocytes and/or CD68 macrophages.
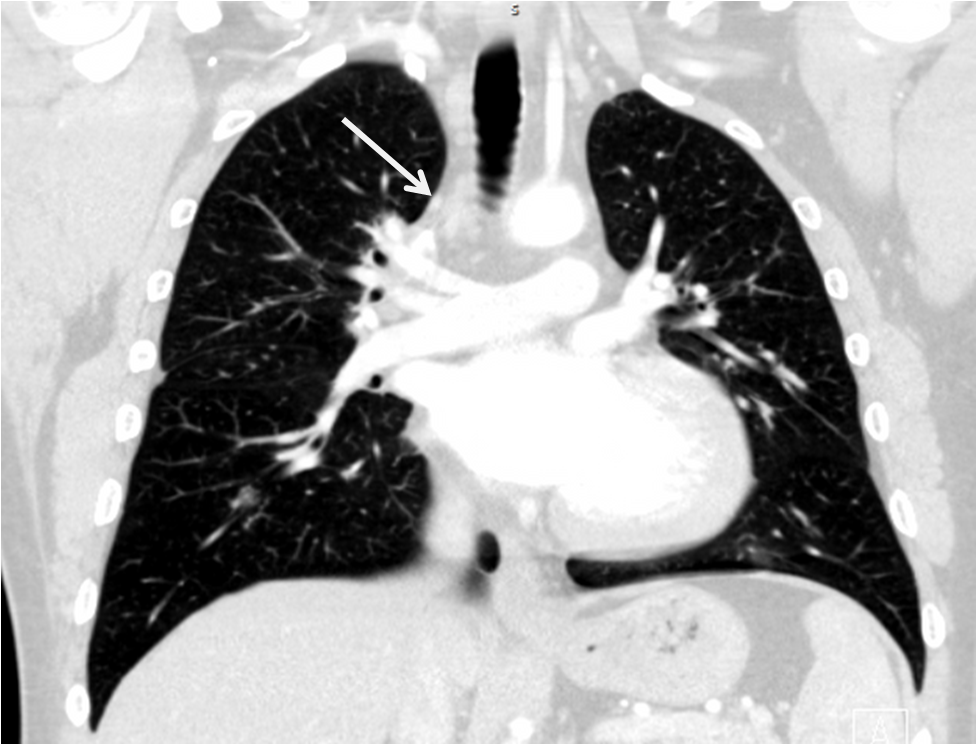
Figure 4:CT scan showing unspecific pulmonary infiltration and mediastinal lymph nodes of borderline size.
After clinical review of the case, in particular the CMR findings, sarcoidosis with cardiac involvement was considered to be the most likely diagnosis. Subsequently, specific therapy with corticosteroids was commenced. In addition, the patient received standard pharmacological heart failure treatment according to current guidelines.
Three months after initiation of medical therapy, a follow-up CMR was performed. Despite optimal medical therapy, neither the functional parameters nor the areas of hyperenhancement showed significant improvement (Movie 3, Figure 5)
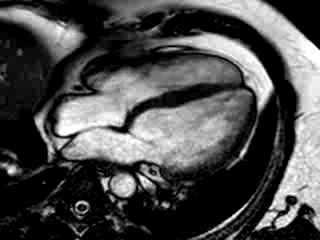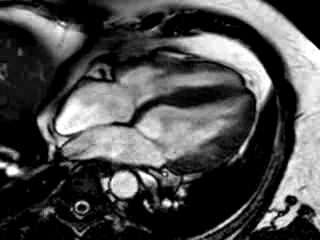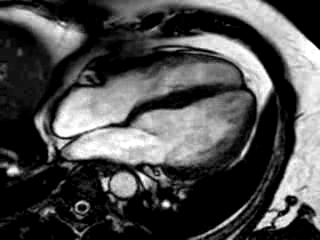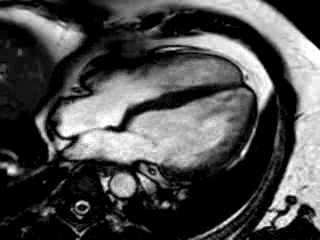
Movie 3: 3 month follow-up. Cine 4-chamber demonstrating a global reduction of LV and RV function with no significant improvement in comparison to the baseline scan
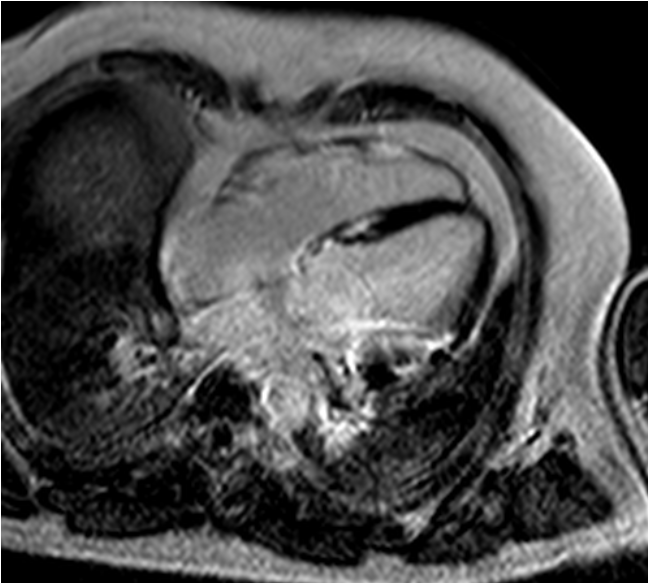
Figure 5 3 month follow-up late-enhancement imaging: 4-chamber view showing no significant reduction of fibrosis.
Further recovery of LV function was therefore considered unlikely especially in the presence of extensive fibrosis. These regions of fibrosis were most likely the cause of multiple polymorphic ventricular ectopies and therefore deemed a potential substrate for further malignant arrhythmias. The patient underwent programmed ventricular stimulation but no ventricular tachycardia could be induced. However, due to extensive fibrosis and impaired LV function, the patient underwent ICD-implantation for primary prevention of sudden cardiac death. Four weeks post-ICD-implantation no episodes of ventricular tachycardia or fibrillation occurred.
Perspective:
The prevalence of cardiac involvement in sarcoidosis ranges from 20% in the US to 60% in Japan and has major prognostic implication.(1,2) Early initiation of specific therapy and device therapy (PPM/ICD) is likely to improve patients’ outcome.
However, the diagnosis of cardiac sarcoidosis can be challenging due to its nonspecific presentation.(3) Clinical manifestations range from complete absence of symptoms to signs of congestive heart failure or conduction abnormalities and ventricular arrhythmias. Myocardial involvement is typically patchy, resulting in low sensitivity of endomyocardial biopsy, around 20-63%. (2,4) Finally, there are no specific laboratory findings for cardiac sarcoidosis.
In contrast to the unspecific clinical and laboratory findings and the low sensitivity of endomyocardial biopsies, cardiac sarcoidosis presents with a characteristic pattern on CMR. Thus, CMR may represent the missing puzzle piece for the diagnosis of cardiac sarcoidosis with subsequent major therapeutic and prognostic impact. Using the only available guidelines for the diagnosis of cardiac sarcoidosis published by the Japanese Ministry of Health and Welfare as reference, CMR showed 100% sensitivity and 78% specificity for the diagnosis of cardiac involvement in sarcoidosis.(5,6) Considering the low sensitivity of endomyocardial biopsies and the high sensitivity and specificity of CMR, further studies are required to redefine the gold-standard for the diagnosis of cardiac sarcoidosis.
References:
1. Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455-469.
2. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204-1211.
3. Doughan AR, Williams BR.Cardiac sarcoidosis. Heart. 2006;92:282-8.
4. Sekiguchi M, Numao Y, Imai M, et al. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980;44:249-63.
5. Hiraga H, Yuwai K, HiroeMet, et al. Guidelines for the Diagnosis of Cardiac Sarcoidosis Study Report on Diffuse Pulmonary Diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare; 1993;23-4.
6. Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683-90.
COTW handling editor: Giovanni Quarta
Have your say: What do you think? Latest posts on this topic from the forum





