Case from: Albert Teis1, Hannah Barnes1, Rory O’Hanlon2, Chiara Bucciarelli-Ducci1.
1Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, London, UK and 2Cardiovascular MRI Unit, St Vincent’s University Hospital, Dublin, Ireland.
Clinical history:
A 79 year old man with a recent episode of non-sustained ventricular tachycardia was admitted to his local hospital for evaluation. ECG demonstrated antero-lateral T-wave inversion (Figure 1). He initially declined to undergo coronary angiogram and was referred for a CMR study as a non-invasive test to assess the presence of inducible ischemia and myocardial viability to guide further management. Previous echocardiography demonstrated mild concentric left ventricular hypertrophy with normal left ventricular (LV) function.
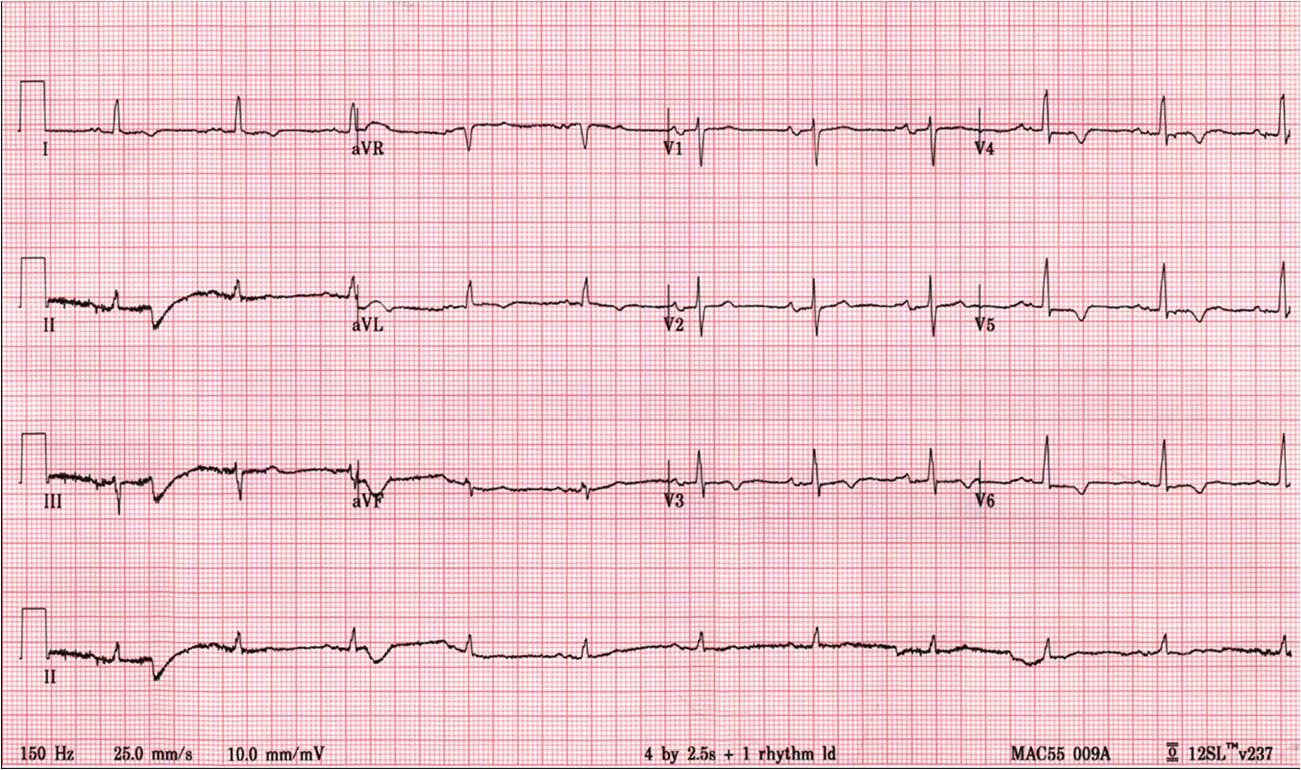
Figure 1
CMR Findings:
Cine CMR images demonstrated a hyperdynamic LV systolic function (EF 84%) with increased LV mass index (87g/m2). Marked apical hypertrophy (12mm) with complete apical LV cavity obliteration in end-systole was noted (Figure 2, Movie 1 and 2).

Figure 2
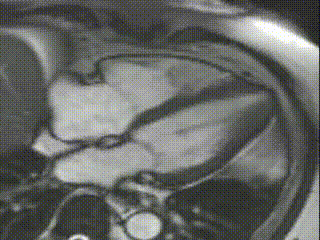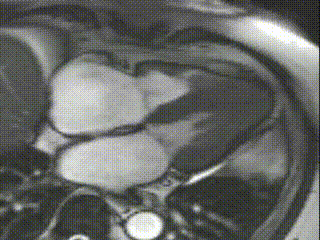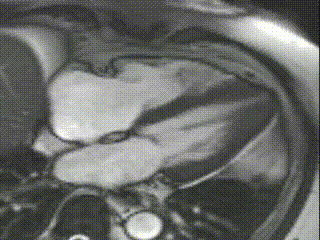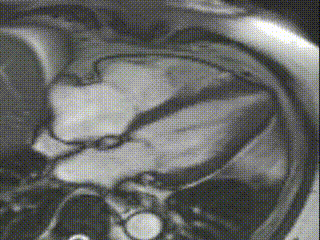
Movie 1
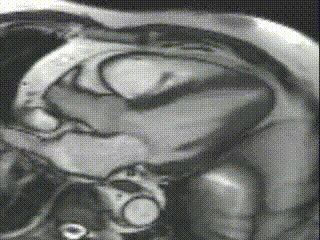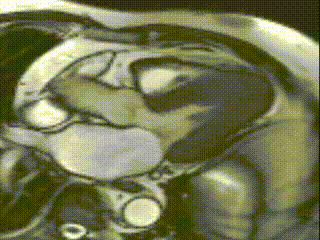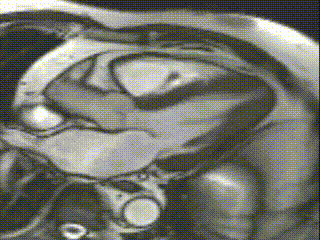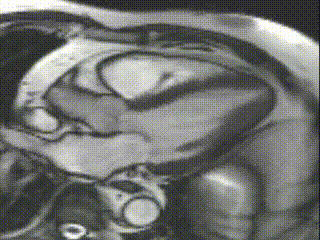
Movie 2
Adenosine stress perfusion demonstrated two distinct patterns of abnormality (Figure 3 and Movie 3): A localised subendocardial perfusion defect in the mid and apical lateral wall was seen, along with a distinct but separate circumferential subendocardial and mid wall defect in the apical hypertrophied regions. Two distinct patterns of scarring/fibrosis were also seen on late gadolinium enhanced (LGE) images: a focal subendocardial infarction in the mid to apical lateral wall and patchy mid wall fibrosis in the apical hypertrophied segments.

Figure 3
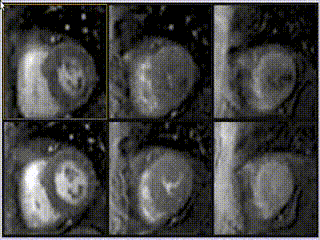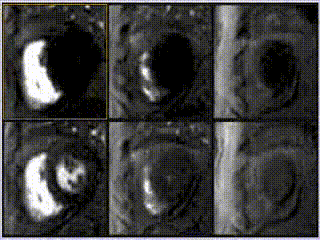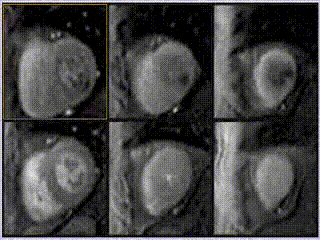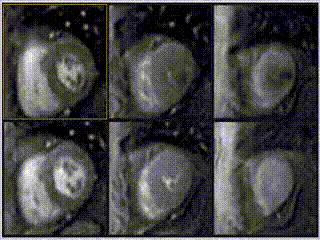
Movie 3
Conclusion:
These two patterns of perfusion abnormalities and LGE suggested dual pathologies. The localised increase in apical wall thickening with associated microvascular dysfunction and mid-wall fibrosis are all hallmarks of apical hypertrophic cardiomyopathy (HCM), whereas the localised infarction of the mid and distal left circumflex (LCx) territory with mild peri-infarct perfusion defect are features of ischemic heart disease (Figure 3, lower row).
The patient subsequently underwent a coronary angiogram. This demonstrated moderate diffuse disease in the left anterior descending and right coronary arteries, and a significant lesion of the mid-distal LCx (Figure 4). Based on the vessel anatomy and on the mild ischemic burden, the patient was managed medically.
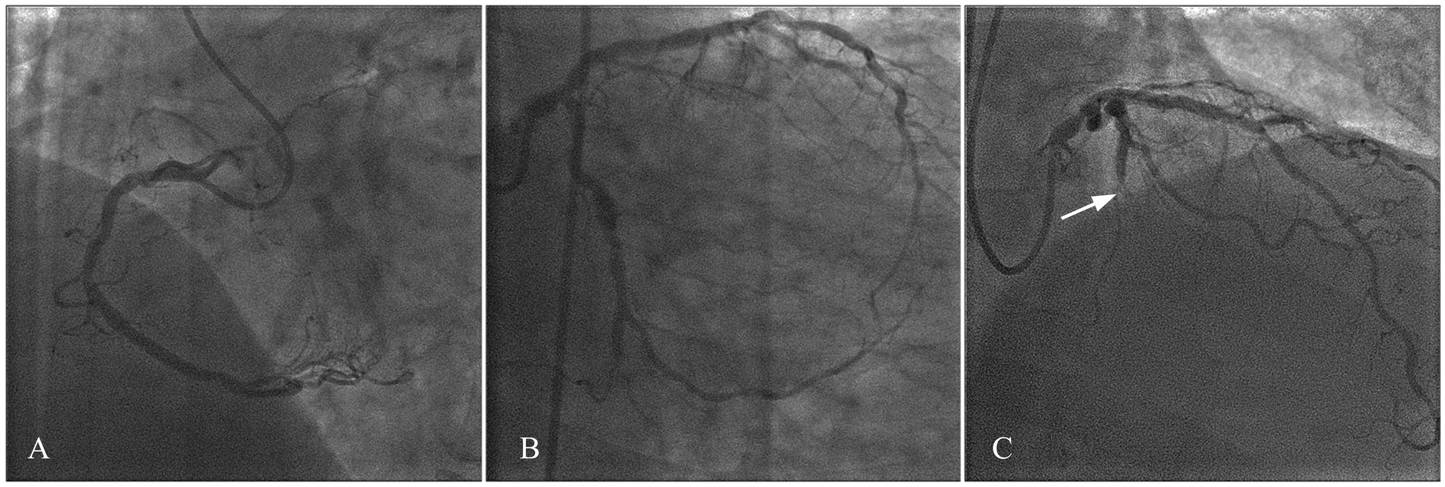
Figure 4
Perspective:
In this case, CMR was key in establishing the final diagnosis of dual pathologies. Apical HCM was a new finding and indeed of importance with regard to prognosis and family screening. The role of CMR to diagnose apical HCM “missed” by echocardiography has been well described and the detection of fibrosis and microvascular ischaemia may be of benefit for risk stratification and prognosis.1,2,3 Furthermore, the role of CMR in assessing burden of ischemia with stress perfusion and viability in ischemic heart disease is also well described. 4,5
In this patient, apical hypertrophy with associated microvascular dysfunction and patchy mid-wall LGE are typical of HCM. The subendocardial infarction with peri-infarct ischemia in the LCx territory confirms ischemic heart disease. However, it remains unclear whether in this case the arrhythmic substrate for the episode of non-sustained ventricular tachycardia was related to fibrosis or ischaemia related to apical HCM or coronary artery disease. Both mechanisms have been described in triggering arrhythmias in ischemic and non-ischemic cardiomyopathies.3,6,7
References:
- Moon JC, Fisher NG, McKenna WJ and Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004;90:645-9.
- Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S and Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric Magnetic Resonance Imaging. Circulation 2007;115:2418-2425.
- Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE and Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J. Am Coll. Cardiol. 2009;54:866-875.
- Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M and Johansson L. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480-9.
- Bucciarelli-Ducci C, Wu E, Lee DC, Holly TA, Klocke FJ and Bonow RO. Contrast-enhanced cardiac magnetic resonance in the evaluation of myocardial infarction and myocardial viability in patients with ischemic heart disease. Curr Probl Cardiol. 2006;31:128-68.
- Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ and Maron MS. Occurrence and frequency of arrhythmias in Hypertrophic Cardiomyopathy in relation to delayed enhancement on Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2008;51:1369-74.
- O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010 Sep 7;56(11):867-74.
COTW handling editor: Vikas K. Rathi







