Case From: Afonso Akio Shiozaki, Fernando Vanzin Rocha, Luciano Henrique Gazoni Scremin, Juliano Lara Fernandes.
Institution: Cardiovascular Magnetic Resonance and Computed Tomography. Section of Maringa Imaging Institute (Instituto Maringa de Imagem) – Brasil.
Case history: A 72 years old woman underwent cardiac magnetic resonance (CMR) in order to investigate possible myocardial ischemia. She had apparently no contraindications to CMR and denied any metal implants.
CMR findings: The first localizer images demonstrated magnetic field artifacts probably due to some metallic device in her superior abdomen. However she again denied any prior abdominal surgery or implantable device (Figure 1A, 1B; movie 1).
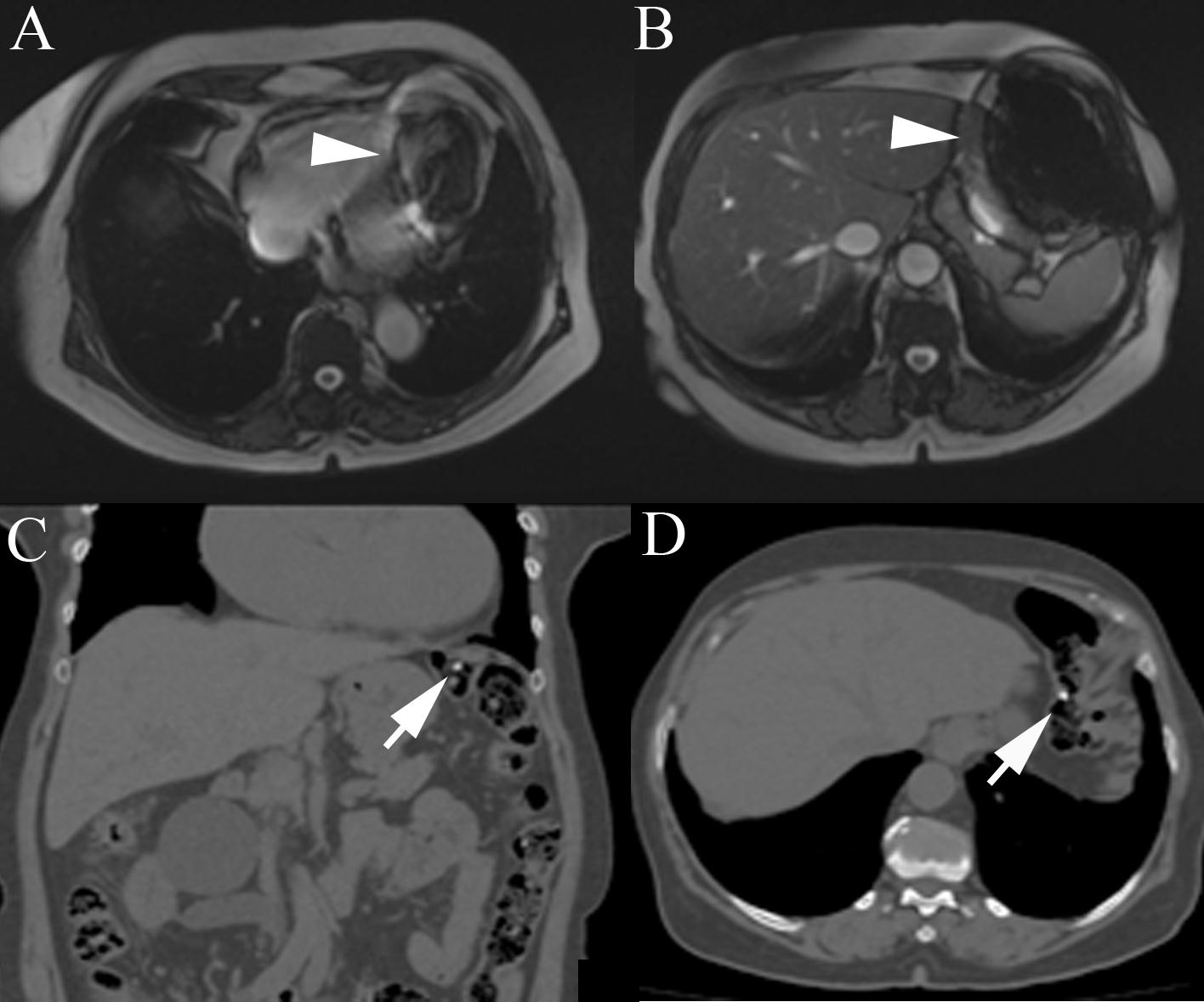
Figure 1
Movie 1
Because of the unidentified problem, we submitted the patient to abdominal computed tomography to search for any metallic device that could justify the resonance images artifacts (Figure 1C, 1D). Computed tomography did not show any metallic devices in her abdomen, however two small high densities capsules were observed inside the transverse colon. She was again asked about food and drugs intake in the last days and at this moment she revealed that she was in treatment of anemia and had taken two ferrous sulfate capsules on the previous day.
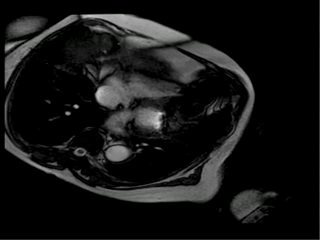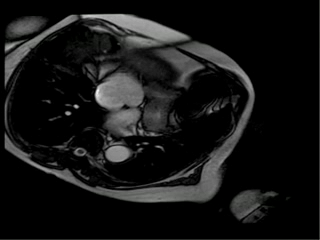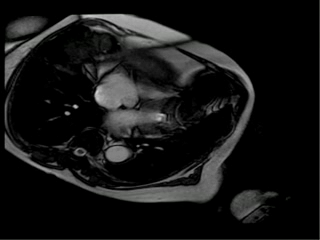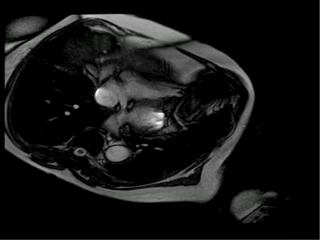
With this new information we suspected that ferrous sulfate capsules could be the cause of the images artifacts on resonance and asked her to return one week after stopping the ferrous sulfate. One week later she underwent cardiac magnetic resonance and the artifact disappeared. In order to confirm that the ferrous sulfate capsules were the cause of artifacts in magnetic resonance images we placed a ferrous capsule used in her anemia treatment on her chest skin and performed some images (Figure 2A-D). Interestingly, the metallic artifacts reappear around the region with the capsules and disappeared when those were removed (Figure 2E, 2F).
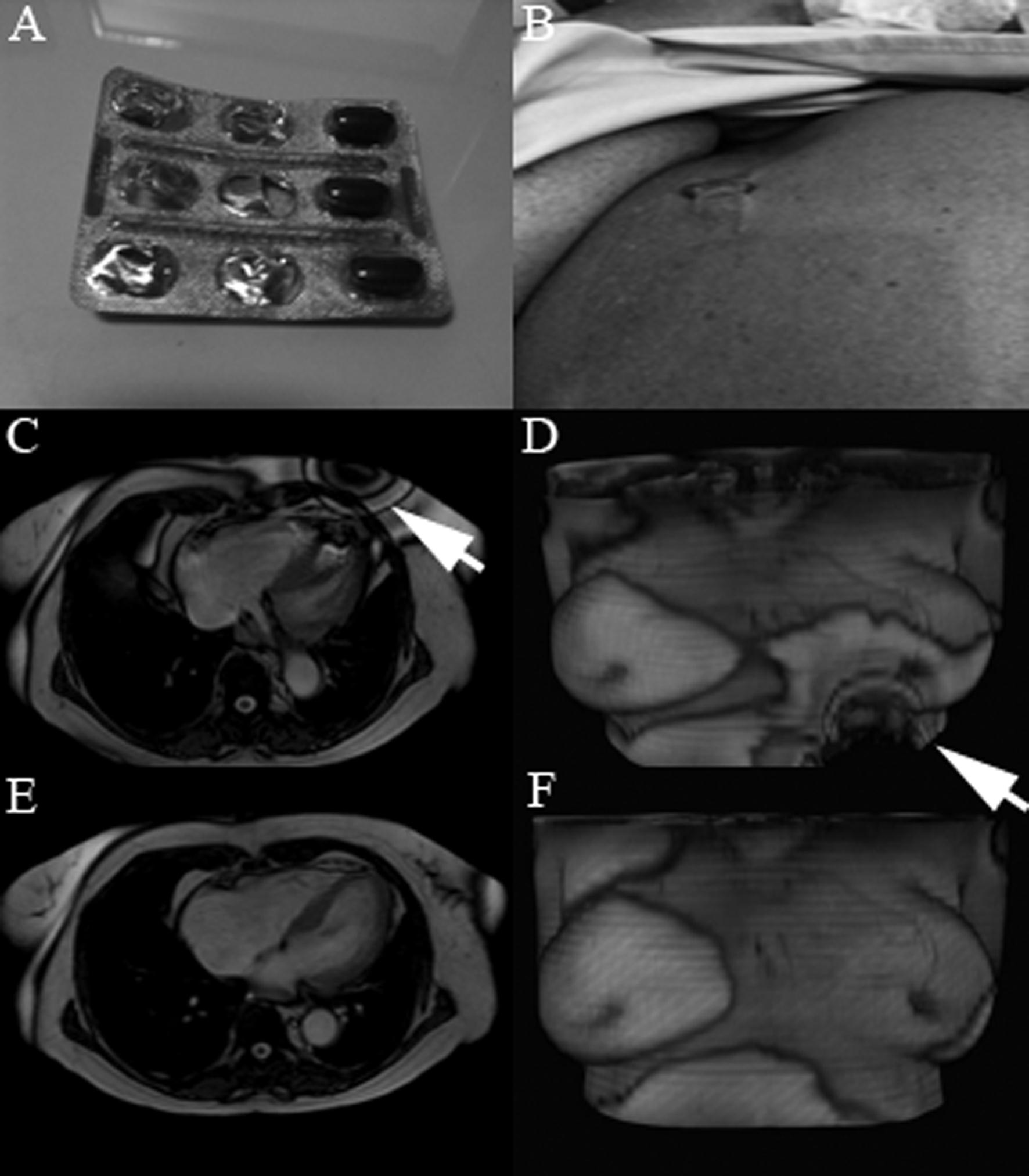
Figure 2
Conclusions: Anemia treatment with ferrous sulfate capsules were not a formal contraindication to magnetic resonance by our knowledge, however the magnetic resonance field disturbance caused by these capsules can significantly impair the quality images of the exam.
Perspectives: Iron deficiency is the most common cause of anemia in the world, especially in underdeveloped countries (1). The association of heart disease and iron-deficiency anemia is fairly prevalent (2) and in patients with heart failure treatment with intravenous ferric carboxymaltose has shown to improve symptoms and functional capacity (3). Therefore, in clinical practice the association of iron supplementation therapy (especially oral supplementation) and heart diseases that are likely to be assessed with CMR is not rare. Ferrous sulphate capsules contain approximately 65mg of iron and have not yet been identified as a source of artifacts for cardiac imaging previously. However, in patients undergoing CMR it should be recommended that the ferrous sulfate should be held at least 24 hours prior to the MRI to allow enough time for the dispersion and total intake of these capsules.
For a similar case on this topic, see Number 07-13: Cine Artefact
References:
1. Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol 2007;17:93-100.
2. Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation 2006;113:2454-2461.
3. Anker SD, Colet JC, Filippatos G et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficieny. N Engl J Med 2009;361:2436-2448.
Case Handling Editor: Vikas K. Rathi, MD
Have your say: What do you think? Latest posts on this topic from the forum







