Case from: Valentina O. Puntmann, MD, PhD1,2; Eike Nagel, MD2, PhD; Britta Butzbach, MD2; Eckart Fleck, MD1; Rolf Gebker, MD, PhD1 Institute: 1 German Heart Institute, 2 Division of Imaging Sciences and Biomedical Engineering, King’s College London, United Kingdom
Clinical history:
We describe two cases with known and previously documented mitral valve (MV) prolapse on transthoracic echocardiography, in line with accepted criteria [1]. Both patients have been referred for a clinical cardiac magnetic resonance (CMR) in independent clinical contexts: for investigation of suspected myocarditis (Case 1, 23 year old female) and angina-like chest pain (Case 2, 72-year old female, hypertensive, on treatment). In both clinical presentations, serial troponin tests were negative (<0.035 mg/mL) and 12-lead ECG showed non-specific ST-changes. In case 1, transthoracic echocardiogram revealed a small pericardial effusion. Both patients underwent CMR protocols in line with their respective clinical indication, which included assessment of LV function with T2 weighted and late gadolinium enhancement imaging in case 1, and a high dose dobutamine-stress testing (DSMR) in case 2.
CMR Findings:
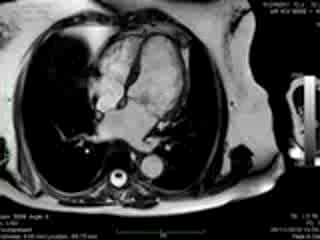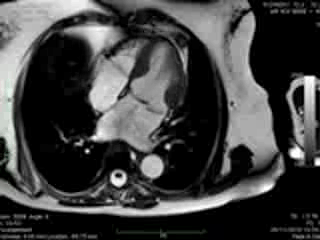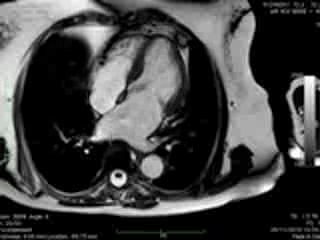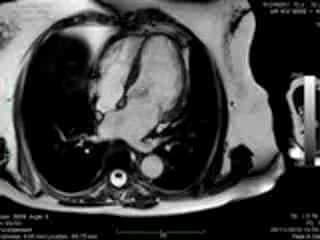
Both cases demonstrated normal biventricular volumes and systolic function with no evidence of late gadolinium enhancement (Figures 1 and 2). Whereas a rim of pericardial effusion was reproduced in case 1, T2 weighted imaging showed no overt increase in regional signal. There was a borderline increase in LV mass (index) in case 2, but no inducible ischaemia on high dose DSMR.
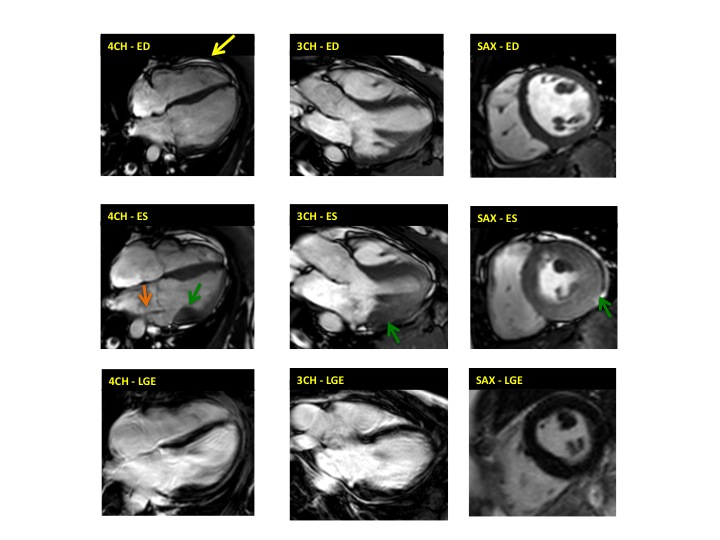
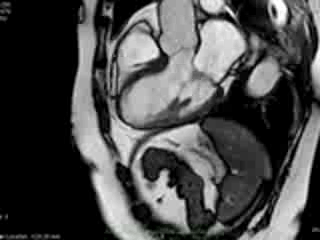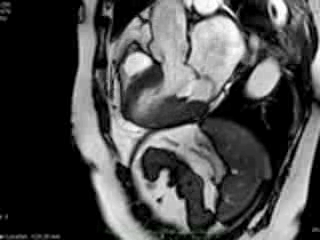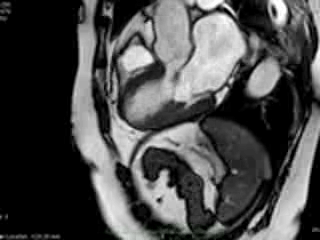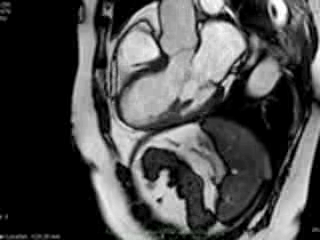
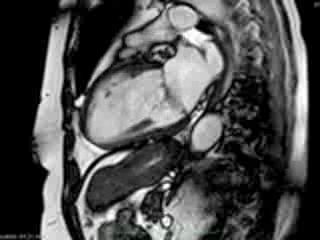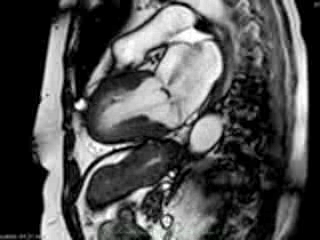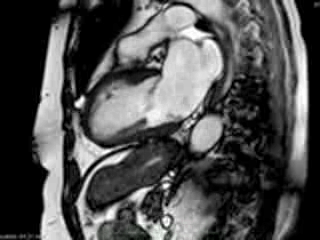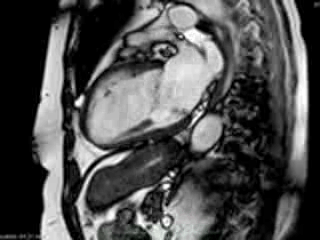
Figure 1. Case 1: 23-year old female underwent CMR in the course of clinically suspected myocarditis. Representative still images in end-diastole (ED) and end-systole (ES). Analysis revealed normal ventricular volumes and ED wall thickness, mildly impaired global systolic function and mild pericardial effusion (yellow arrow). Note an intense regional contraction in the inferolateral basal ventricular segments (green arrows) and mid-systolic mitral regurgitation (orange arrows). No late gadolinium enhancement (LGE)
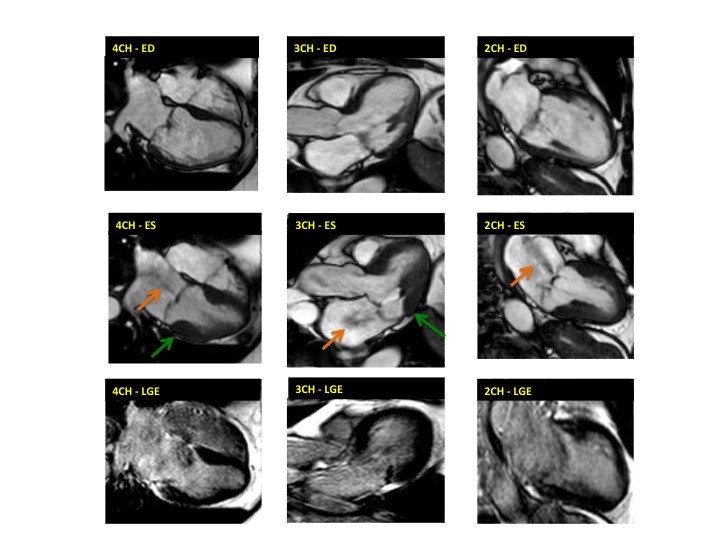
Figure 2. Case 2: This 72-year old female was referred for investigation of atypical chest pain and underwent dobutamine-stress CMR (no inducible ischaemia). Representative ED and ES still images. Note is made of an intense contraction in the inferolateral basal (BA) ventricular segments (green arrows) and mid-systolic mitral regurgitation (orange arrows). No LGE.
both, cine imaging revealed prolapse of posterior MV leaflet with mitral regurgitation (Case 1: Movies 1-3, Case 2: Movies 4-7). Careful consideration of inferior and inferolateral segments revealed increased early-mid systolic thickening in basal segment with apical displacement of inferolateral aspect of MV annular plane, coinciding with the onset of mitral regurgitation. We used feature-tracking post-processing approach cine-images and demonstrated that in both cases differential regional deformation leads to apical displacement of inferolateral MV annulus and precedes the onset of mitral regurgitation (Figures 3,4).
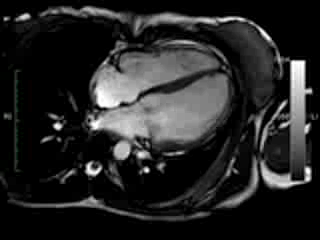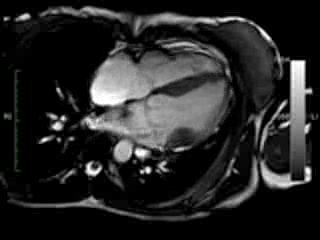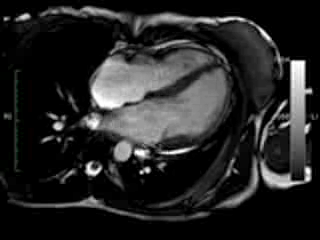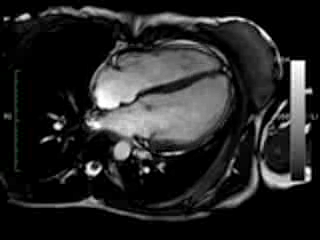
Movie 1 & Movie 2
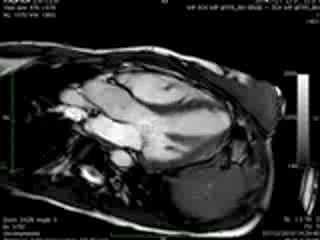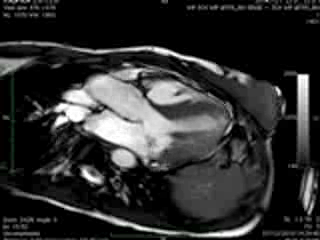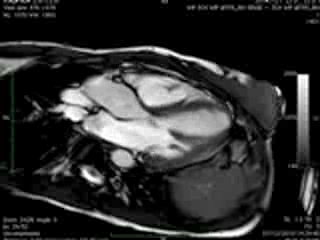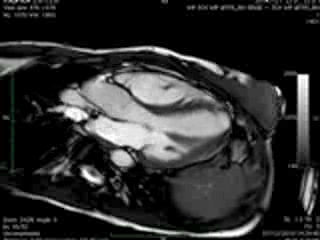
Movie 3 & Movie 4
Movie 5 & Movie 6
Movie 7
Representative cine-loops of short axis, 2-,3-, and 4-CH views of case 1 (Movies 1-3) and case 2 (Movies 4-7) reveal increased regional contraction in the basal inferolateral segments leading to apical displacement of the posterior portion of the mitral annulus with a mid-systolic onset of regurgitant flow
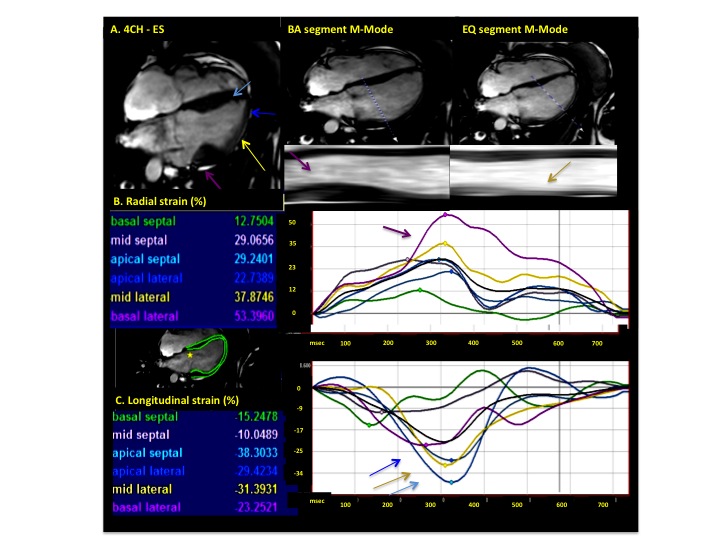
Figure 3. Analysis of myocardial deformation in 4-chamber view (Case 1). Color-matched arrows and time-resolved strain curves correspond to the adjacent legend. Cine and M-Mode images reveal increased ES thickening in BA segment in comparison to equatorial and apical segments (A). Note increased radial systolic strain values in basal lateral segment (purple arrow, B) and increased longitudinal strain in mid-lateral and apical segments (blue and yellow arrows, C)
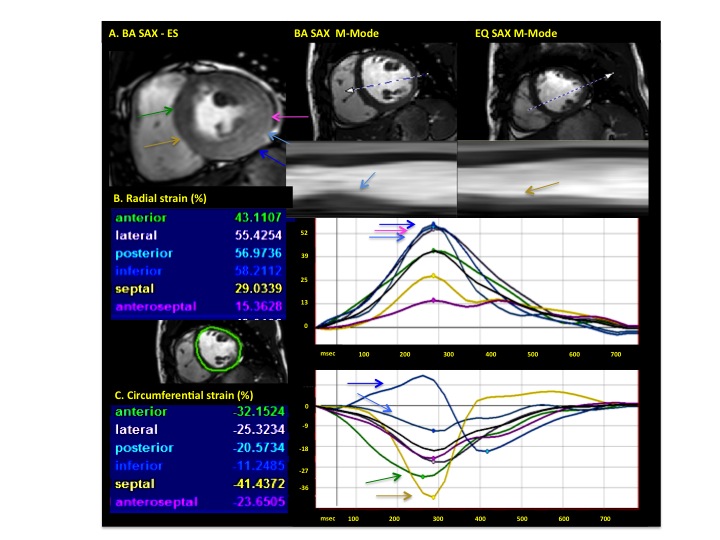
Figure 4. Analysis of myocardial deformation in short-axis (SAX) view (Case 1). Representative M-Mode in BA and equatorial slice (A), revealing increased thickening in the basal lateral wall. Note is made of increased radial and decreased circumferential strains in the lateral and inferolateral segments (blue and pink arrows, B), whereas the anteroseptal segments show increased circumferential strain values (C)
Conclusion:
Pathophysiologically, MV prolapse is regarded as an intrinsic abnormality of the MV and its subvalvular apparatus, characterized by displacement of MV leaflets from the annular plane, causing various degrees of regurgitation during systole [1]. Our observations indicate a possible novel mechanism outlined by differential (basal- apical) myocardial deformation in inferior and inferolateral walls leading to apical displacement of the MV annular plane, relative displacement of the posterior leaflet below the plane of the anterior leaflet and consequently mitral regurgitation. We reveal such mechanism in two independent clinical cases of known MV prolapse, potentially summing up to a novel observation warranting further mechanistic studies by assessment of myocardial deformation.
Perspective:
Echocardiography is regarded as the preferred choice imaging modality in assessment of this condition due to the excellent valvular views [1,2]. However, a narrow field of view and a strong focus on the valvular area may lead to a limited appreciation of the myocardium. Consequently, important pathophysiological insights may go unnoticed, as the ventricle is not the primary focus. CMR is conventionally considered less apt for valvular assessment [3], however, in the present contribution we demonstrate its role in providing complementary information by integrating myocardial assessment. Moreover, we provide for the first time an insight into the pathophysiological mechanism leading to displacement of the posterior MV leaflet by differential myocardial deformation in two independent cases with known MV prolapse. Whether the hyperdynamic basal inferolateral wall is the cause of MV prolapse or an unrealted observation, remains to be confirmed. Because we support our observations with time-resolved strain curves, which correspond with M-Mode displays of increased myocardial thickening, we suggest that our account identifies a possible mechanistic explanation within the myocardium, which may be partially explained by MV annular dilatation, if present. Prospective imaging studies in representative patients samples are warranted to confirm such findings into a distinct imaging signature, reveal insights into the causes, and guide towards a possible treatment.
Acknowledgement We acknowledge the support of TomTec GmbH, Germany, for providing us with the feature-tracking 2D CPA MR prototype software
References:
1. Bonow RO, Carbello BA, Chatterjee K et al. ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease. J Am Coll Cardiol. 2006;48:e1-e148
2. Biaggi P, Gruner C, Jedrzkiewicz S, Karski J, Meineri M, Vegas A, David TE, Woo A, Rakowski H. Assessment of mitral valve prolapseby 3D TEE angled views are key. JACC Cardiovasc Imaging. 2011;4:94-7.
3. Herbots L, Maes F, D’hooge J, Claus P, Dymarkowski S, Mertens P, Mortelmans L, Bijnens B, Bogaert J, Rademakers FE, Sutherland GR. Quantifying myocardial deformation throughout the cardiac cycle: a comparison of ultrasound strain rate, grey-scale M-mode and magnetic resonance imaging. Ultrasound Med Biol. 2004;30:591-598.
4. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3(2):144-51.
COTW handling editor: M. Jay Campbell, MD
Have your say: What do you think? Latest posts on this topic from the forum







