Case from:Arash Seratnahaei, Kunal Bodiwala, Steve W. Leung, Vincent L. Sorrell
Institute: University of Kentucky Medical Center, Division of Cardiovascular Medicine, Gill Heart Institute; Lexington, KY
Clinical history: A 23 year-old morbidly obese (BMI=51 kg/m2) Caucasian female with Down Syndrome was born with double outlet right ventricle (DORV), complete atrioventricular septal defect (CAVSD), and pulmonary stenosis. At age 5, she underwent complete surgical repair of the CAVSD, DORV, including patch pulmonary valvotomy, and mitral valve repair at another institution. She also has a reported history of myocarditis in 2007 with resultant systolic dysfunction (EF 35-40%). She was referred to our institution for dyspnea on exertion. She was on standard congestive heart failure therapy.
Conventional Imaging:
Transthoracic echocardiogram (TTE) findings: Her TTE was technically difficult due to her body habitus. She had moderate pulmonary regurgitation (Movie 1) with severe pulmonary stenosis (peak gradient 65 mmHg, mean gradient 37 mmHg, peak velocity 4 m/s) (Image 1), evidence of CAVSD repair without residual AVSD (Movies 2, 3, and 4), mildly reduced right ventricular systolic function and left ventricular global systolic function of 30-40% (Movies 2 and 4). There were no segmental wall motion abnormalities identified on TTE.

Movie 1. Parasternal short axis color Doppler focusing on the pulmonary valve showing flow acceleration across pulmonary valve, at least moderate pulmonary regurgitation, difficult to visualize the leaflets.
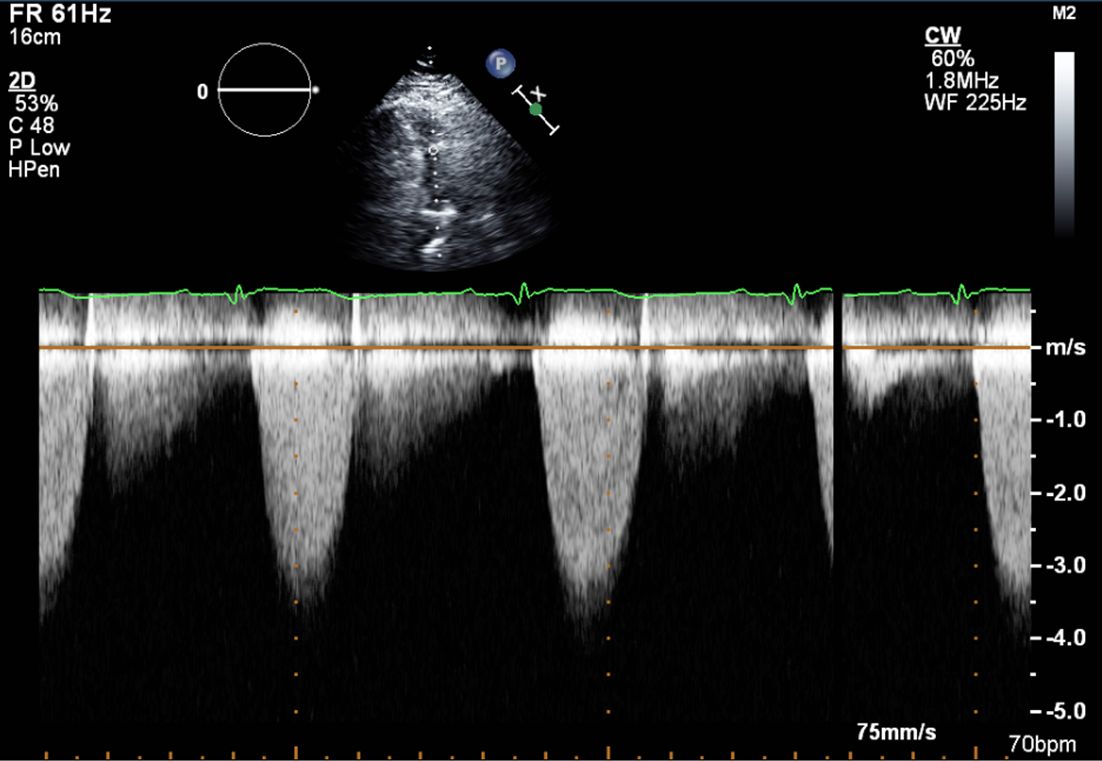
Image 1. Continuous wave Doppler through the pulmonary valve. Peak velocity 4 m/s, peak gradient 65 mmHg consistent with severe pulmonary stenosis.

Movie 2. Parasternal long axis view, showing dilated right ventricular outflow tract (RVOT), echo bright structure in the left ventricular outflow tract (LVOT) area suggesting a patch repair of ventricular septal defect (VSD).
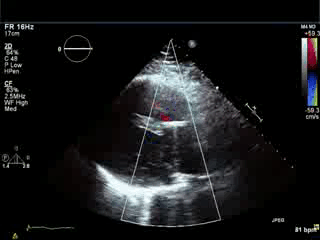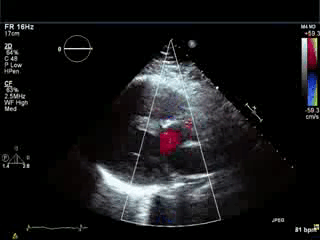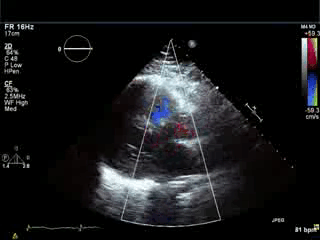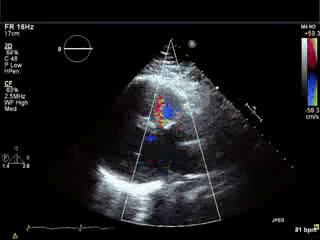
Movie 3. Parasternal long axis color Doppler showing flow during diastole in the RVOT area from pulmonary regurgitation and no residual evidence of left-to-right shunt in the area of VSD patch.
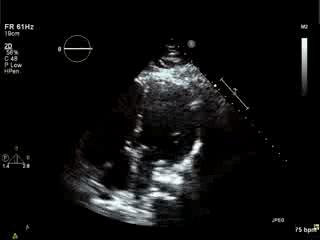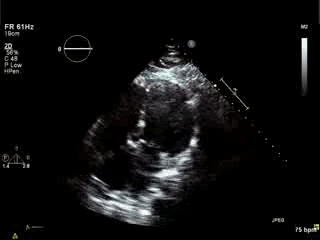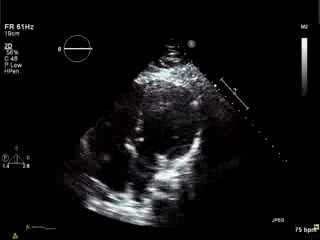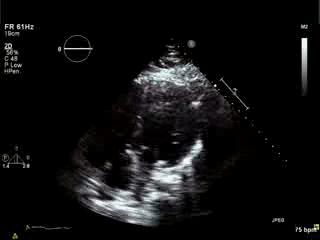
Movie 4. Apical 4 chamber view demonstrating moderately dilated right ventricle (RV), reduced right ventricular and left ventricular (LV) global systolic function. The VSD patch repair is also seen.
Treatment based on TTE findings alone: There is no specific medical therapy for valvular pulmonary stenosis. If right-sided heart failure occurs, it is treated primarily with diuretics. Based on the TTE findings alone, the treatment of significant pulmonary stenosis is by balloon valvuloplasty or surgical intervention.1 Percutaneous pulmonary valve placement can be an option for certain patients with pulmonary regurgitation and right ventricular outflow tract obstruction.2
Invasive Imaging:
Right Ventriculography findings: Right heart catheterization (RHC) and right ventriculography was performed to evaluate the severity of pulmonary stenosis and potential pulmonary hypertension. The RHC revealed no intracardiac left-to-right shunt, moderate pulmonary hypertension (RA 14 mmHg, pulmonary artery pressure 60/23, mean 36 mmHg, pulmonary capillary wedge pressure 14 mmHg, calculated pulmonary vascular resistance 4.2 Wood Units), mild pulmonary stenosis (peak gradient 33 mmHg), and normal cardiac output 5.3 L/min and cardiac index 3.0 L/min/m2. Right ventriculography revealed normal blood flow to the right and left pulmonary arteries, mildly enlarged RV with near normal right ventricular systolic function (Movie 5). However, quantitative analysis of the RVOT, pulmonary artery and branch pulmonary arteries were limited due to concerns about required increased contrast and radiation exposure.

Movie 5. Right ventriculography in AP projection showing mildly enlarged RV, near normal RV function, moderate-appearing pulmonary stenosis and patent pulmonary arteries.
Treatment based on RHC: Based on the RHC findings, the patient would have been treated medically for mild pulmonary stenosis and would have avoided a surgical procedure based solely on TTE findings.
CMR Findings: The patient was referred for CMR for further anatomic characterization and RV volume and function quantification. There was moderate pulmonary regurgitation (regurgitation fraction 21%) (Movie 6), borderline RV systolic function (RV EF 44%), borderline elevated RV volume (RVEDVI 93 ml/m2), mild mitral regurgitation (regurgitant fraction 16%, calculated by (SV [volumetric method] – Qs [phase contrast method])/SV, no aortic regurgitation was present), and moderately reduced LV systolic function (LV EF 38%) (Movies 7 and 8). CMR showed intact patch repair of the interatrial septum and interventricular septum consistent with her previous surgical history (Movies 8 and 9). Time-resolved magnetic resonance angiography (MRA) showed no evidence of pulmonary artery stenosis or aneurysm (Movie 10). Accurate 3D assessment of the RVOT, pulmonary artery and branch pulmonary arteries were performed, which could not be performed by standard invasive RV angiography. (Movie 11). There was no shunt (Qp:Qs = 1.0). Near transmural delayed enhancement involving the epicardium in the mid inferior wall extending into basal and inferolateral wall was evident on late gadolininum enhancement (LGE) (Images 2A-C).
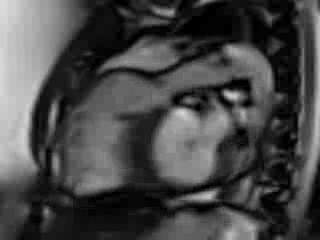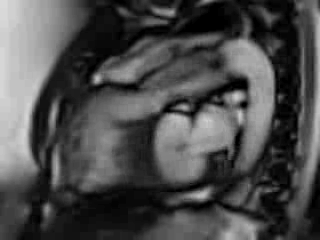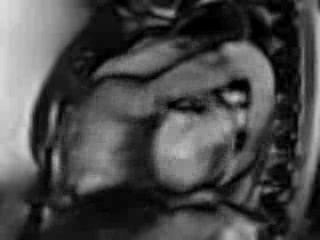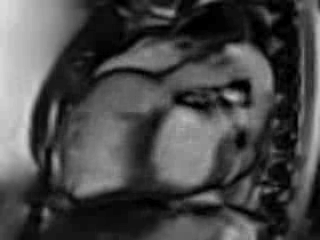
Movie 6. Free breathing cine Steady State Free Precession (SSFP) RVOT view showing a patch in the RVOT with a discrete narrowing at the level of pulmonary valve with flow acceleration across the valve and pulmonary regurgitation
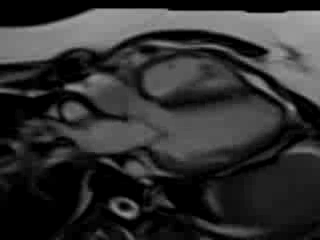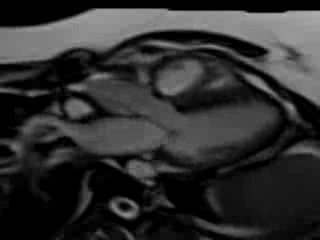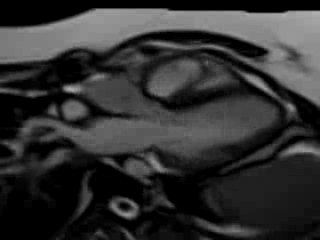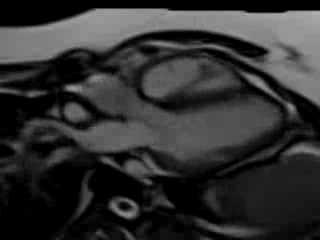
Movie 7. Free breathing cine SSFP 3 chamber view showing moderately reduced LV function with inferolateral wall hypokinesis.

Movie 8. Free breathing cine SSFP 4 chamber view showing moderately reduced LV function, mild mitral regurgitation, and a patch in the atrioventricular septal area
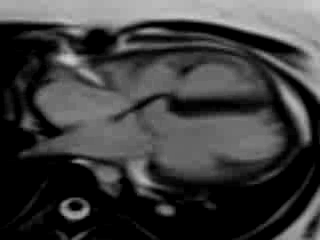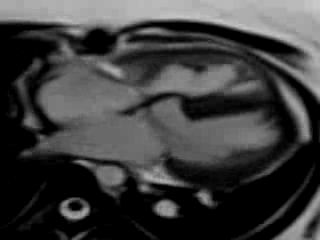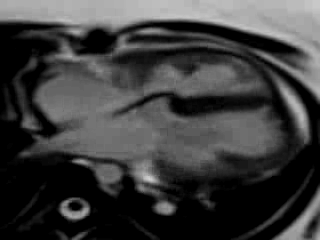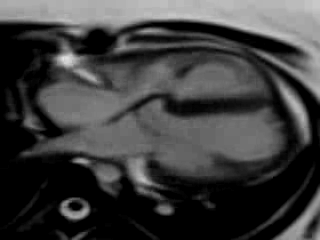
Movie 9. Free breathing cine SSFP axial image showing the patch in the atrioventricular septum consistent with her prior surgical repair of AVSD

Movie 10. MRA showing no evidence of pulmonary artery stenosis or aneurysm
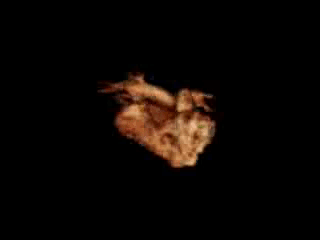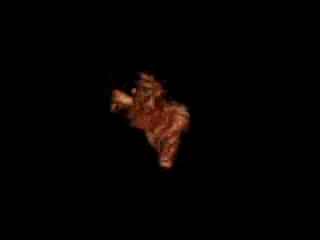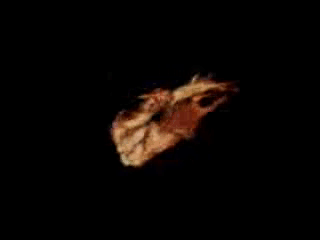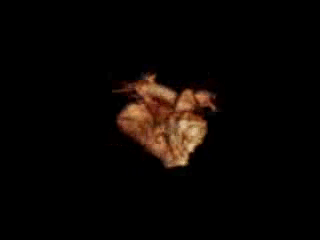
Movie 11. 3D MRA reconstruction of RVOT, pulmonary artery and pulmonary artery branches can be assessed on advanced work station with 3D analysis and accurate measurement of diameter and length of RVOT and pulmonary arteries.
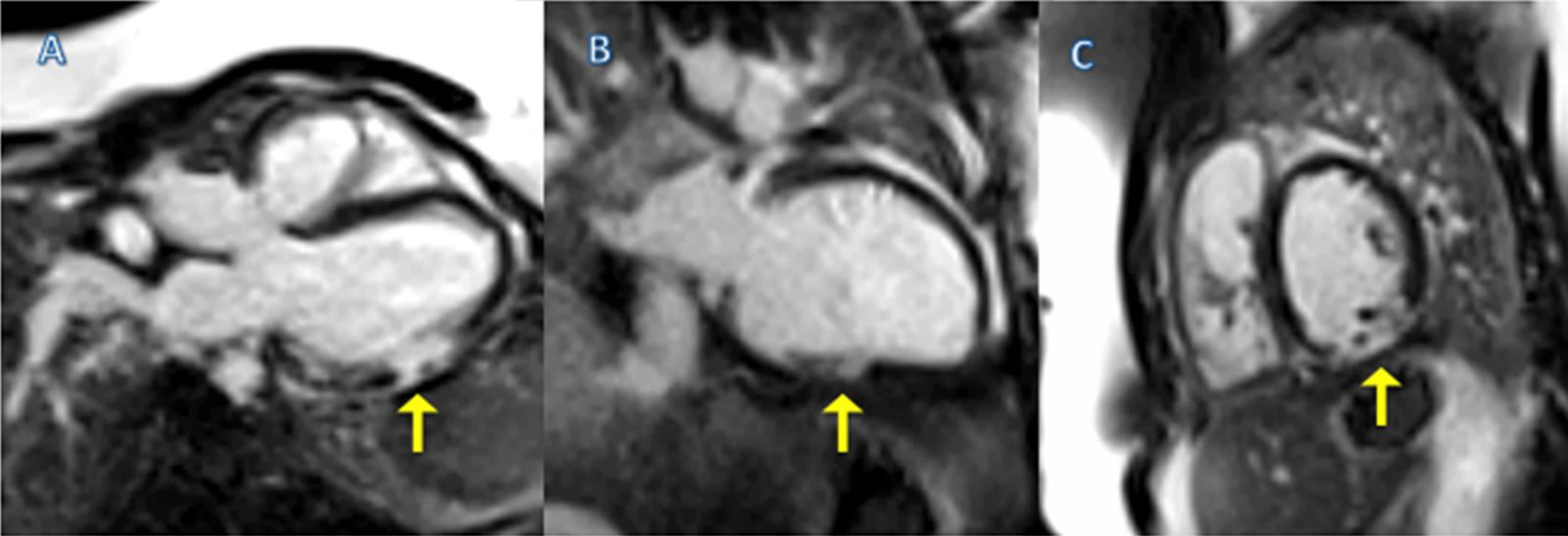
Images 2A-C. Single-shot images showing near transmural delayed enhancement involving the epicardium LGE in the mid inferior wall extending into basal and inferolateral wall (arrow), correlating with her prior history of myocarditis. This area of wall motion abnormality was difficult to assess by echocardiography.
Conclusion: It is known that in patients with left-dominant or codominant coronary anatomy, the circumflex artery courses near the mitral annulus and, thus, is susceptible to injury during mitral valve surgery. Review of the published literature, as well as our own clinical experience, (presumably related to the proximal site of coronary artery injury), suggests that most of these patients have extensive myocardial infarctions and complicated peri-operative clinical course. Although a peri-procedural injury cannot be entirely excluded, the available clinical history as well as the isolated, mid-lateral wall injury, seem to support a diagnosis of myocarditis.
TTE is first-line imaging modality in adults with congenital heart disease.3 Though the TTE provided information regarding many of the pathological findings, it was not able to adequately quantify the RV chamber size and function which is needed to decide the type and timing of potential surgical interventions.4 TTE is also unable to consistently provide tissue characterization. Thus, the multimodal approach to complex patients with potential multiple pathologic processes is vital.
CMR provided excellent image quality despite morbid obesity and free breathing. CMR demonstrated regional inferolateral wall motion abnormality that was missed by TTE. Near transmural LGE involving the epicardium of the inferior and inferolateral wall is consistent with the reported history of myocarditis, although we realize that a left circumflex artery (or branch) injury with resultant myocardial infarction during her surgical repair cannot be entirely excluded. MRA provided superior quality than the invasive angiography without exposing the patient to the risks of the invasive procedure and radiation. The patient will be treated medically based on CMR findings of mild pulmonary stenosis, moderate pulmonary regurgitation, and mild to moderate biventricular systolic dysfunction which all likely contributed to her dyspnea. Weight loss was also encouraged. There was no evidence of residual shunt (Qp:Qs = 1.0).
Perspective: Patients with Down Syndrome have a higher rate of congenital heart disease than the general population. Nearly 44% are born with one or more cardiac defects. In particular, AVSD is particularly characteristic in the Down Syndrome population.5 With the increasing longevity of patients with corrected complex congenital heart disease, noninvasive imaging plays an important role in clinical decision making. CMR provides characterization of the anatomy, physiology and evaluation of ventricular function in this population.6 It also helps to detect those who need intervention again.7 Evaluation of right and left ventricular function is vital in patients with corrected congenital heart disease.8 CMR is considered the standard reference for RV size, geometry and function measurements.9 As demonstrated in this case, modern CMR scanners have the ability to provide high quality images despite uncontrollable factors such as patient obesity and free breathing.
References:
1. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease); American Society of Echocardiography; Heart Rhythm Society; International Society for Adult Congenital Heart Disease; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 Dec 2;52(23):e143-263.
2. McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010 Aug 3;122(5):507-16.
3. Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J. 2010 Apr;31(7):794-805.
4. Kilner PJ. Imaging congenital heart disease in adults. Br J Radiol. 2011 Dec;84 Spec No 3:S258-68.
5. Freeman SB, Bean LH, Allen EG, Tinker SW, Locke AE, Druschel C, Hobbs CA, Romitti PA, Royle MH, Torfs CP, Dooley KJ, Sherman SL. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. 2008 Mar;10(3):173-80.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010 Jun 8;121(22):2462-508.
7. van der Hulst AE, Roest AA, Westenberg JJ, Kroft LJ, de Roos A. Cardiac MRI in postoperative congenital heart disease patients. J Magn Reson Imaging. 2012 Sep;36(3):511-28.
8. Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004 Mar 17;43(6):1068-74.
9. Crean AM, Maredia N, Ballard G, Menezes R, Wharton G, Forster J, Greenwood JP, Thomson JD. 3D Echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J Cardiovasc Magn Reson. 2011 Dec 8;13:78.
COTW handling editor: Sohrab Fratz, MD, PhD
Have your say: What do you think? Latest posts on this topic from the forum







