Case from: Lilia M. Sierra-Galan1, Gilberto Gomez-Garza2, Ricardo García-Buen-Abad2, Julio Lopez-Cuellar1, Alejandro Rey-Rodriguez1, Eulo Lupi-Herrera1.
Institute: 1Cardiovascular and 2Imaging Divisions, American British Cowdray Medical Center, Mexico City, Mexico.
Clinical history:
A 16 year old male previously dilated from congenital post-ductal aortic coarctation (AoCo) through balloon angioplasty at age of 1 year-old, was referred to cardiovascular imaging to rule out any possible complications derived from high-performance and continuous physical activity as a professional soccer player; the patient had an athletic constitution and alluded to be asymptomatic, except for the presence of sporadic headaches after prolonged training periods. The physical examinations have always been unremarkable and no murmurs were reported.
The systemic blood pressure at rest was normal, and during treadmill stress test it behaved with a hypertensive response tendency but still within the upper limits of normal range according to age and gender, reaching 156/90 mmHg at peak exercise, with slow normalization of blood pressure during the following 6 minutes of the recovery period. The heart rate reached the maximum expected for age with almost immediate recovery to basal rate within the first minute of post-exercise period.
Based on the ESC guidelines1, the gradient should be > 20 mmHg with upper limb hypertension to be considered as significative; in this case, his brachial-ankle index at rest was 25 mmHg with normal systemic blood pressure on his upper limb; therefore not fulfilling this criteria. EKG was normal. Ambulatory blood pressure monitoring was not performed.
Considering the high risk of comorbidities and/or complications during continuous high-performance exercise for a patient treated from AoCo,2-6 we considered the measurement of the hemodynamic response to stress could be clinically relevant.
The transthoracic echocardiogram (TTE) with physical exercise could be an option. However, during the test in this scenario, the dynamic gradient is not accurately measured due to the patient’s excellent physical condition, his rapid recovery (<1 minute), and due to TTE technical limitations on acquisition of detailed images during the maximal effort.7-8
Hence, we considered the use dobutamine, under a strictly controlled and safe environment, to be justified in order to accurately measure the hemodynamic response to stress in a patient with such specific characteristics.
We decided to perform a dobutamine stress cardiovascular magnetic resonance (DSCMR), with acquisition of steady state free precession (SSFP) sequences of the heart in short and long axis views, main long axis of 2, 3 and 4 chambers views, and a sagittal plane of the aorta; also obtained SSFP and transverse phase contrast (PhC) sequences of the ascending aorta and just below the narrowed segment before and during dobutamine induced stress using a currently accepted protocol. 9
CMR Findings:
CMR findings of the heart were normal (Movies 1 and 2). The anatomy of the aortic arch was normal, there was a right brachiocephalic trunk from which right carotid and right subclavian arteries originate and independent origins of left carotid and left subclavian arteries. The narrowed segment was distal to the origin of the left subclavian artery.
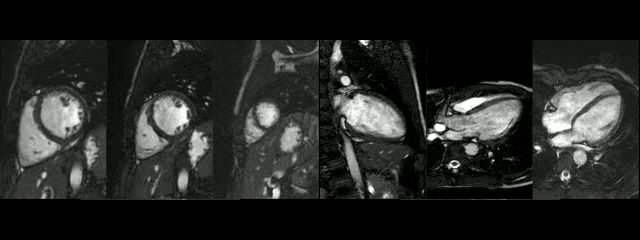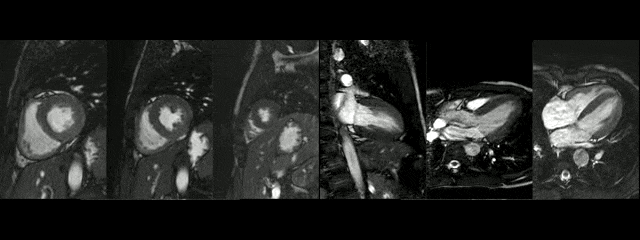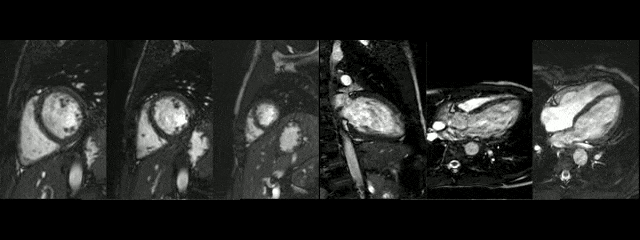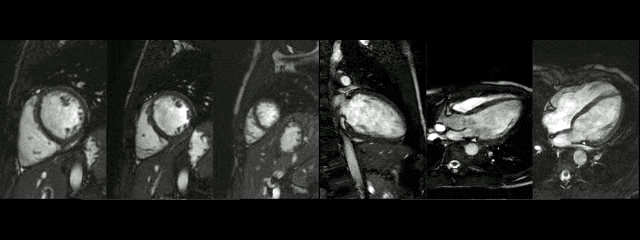
The diameters of the aorta on different segments were also on normal range and the narrowed portion was not significant (Table 1).
| Aortic segment | * Normal values (range) | Diameter(Index) |
|---|---|---|
| Sino-tubular junction (mm) | 24.9(18.1 – 31.7) | 23(18) |
| Ascending aorta (mm) | 26.6(18.2 – 35.0) | 21(16) |
| Proximal descending aorta (pre-coarctation) (mm) | 20.4(14.6 – 26.2) | 17(13) |
| Coarctation (mm) | — | 16(12) |
| Proximal descending aorta (post-coarctation) (mm) | 20.4(14.6 – 26.2) | 22(17) |
Table 1. Aortic diameters and corresponding indexes to the BSA (1.29m2). * 20
During rest, SSFP and PhC sequences of aorta showed mild acceleration blood flow through the stenosed segment. Measurements were taken 8 mm below the narrowest segment and axial to the flow, where the peak velocity was 2.52 m/sec, with a dynamic gradient calculated in 25 mmHg (Figure 1, Movie 3).
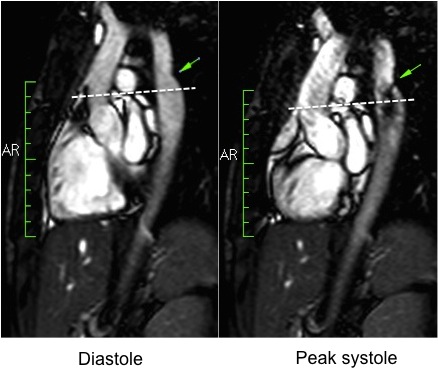
Figure 1. SSFP and PhC of aorta (sagittal and transverse plane). SSFP sagittal plane of the aorta at rest in diastole and peak systole, showing mildly reduced diameter at the narrowest level (arrow) and turbulent flow under the AoCo area. The dashed line shows the level where the measurements were taken just below the narrowest segment in the descending aorta for the peak velocities and flow, and the measurement of flow in the ascending aorta.
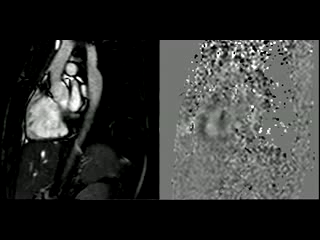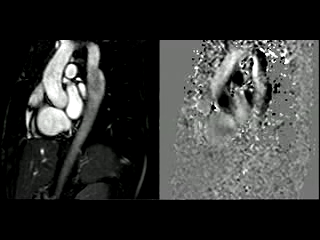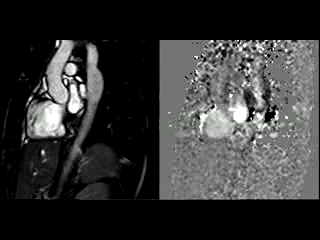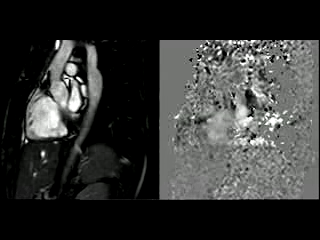
During the maximum stress, considered as >85% of maximal heart rate expected for the given age and the cardiac output almost triplicated, induced by dobutamine infusion at 40 mcg/kg/min plus 1 mg of atropine (following the standard protocol9 and taking the corresponding safety measurements), the peak velocity measured, at the same location as in rest, was of 3.55 m/sec, which resulted in a gradient of 50 mmHg (Table 2 and Figure 1). There were no side effects or complications during the test.
| CMR results (index) | Rest | Stress* |
|---|---|---|
| CO (L/min) | 5.4 (4.2) | 14.4** (11.1) |
| HR (bpm) | 76 | 199** |
| LVEF (%) | 64 | 67 |
| CDG (mmHg) | 25 | 50** |
| SV (mL) | 73 (57) | 74 (57) |
| LVEDV (mL) | 116 (90) | 110 (85) |
| LVESV (mL) | 42 (33) | 36 (28) |
| LV Mass (g) | 98 (74) | — |
| LBBP (mmHg) | 102/50 | 139/67** |
| LABP (mmHg) | 123/52 | 145/87** |
| ABPI (mmHg) | 21/2 | 6/20** |
*Dobutamine 40 mcg/kg/min + 1 mg Atropine. ** <0.05
Abbreviations: CMR: cardiovascular magnetic resonance, CO: cardiac output, HR: heart rate, LVEF: Left ventricular ejection fraction, CDG: calculated dynamic gradient, SV: Systolic volume, LVEDV: left ventricular end-diastolic volume, LVESV: left end-systolic volume, LV Mass: Left ventricular mass, LBBP: left brachial blood pressure, LABP: left ankle blood pressure, ABPI: Ankle-Brachial pressure index.
The flow-time curves at rest showed no diastolic tail7, indicating no significant coarctation, however during maximum induced stress a diastolic tail in the descending aorta becomes evident distal to the mildly narrowed segment, suggesting that during stress it is hemodinamically significant (Figure 2).
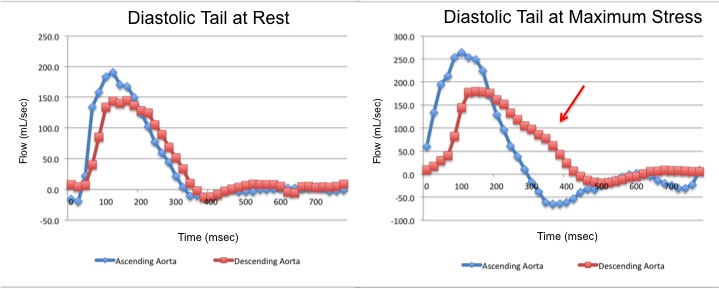
Figure 2. Flow-time curves at rest and at maximum induced stress. Graph at rest shows there is no diastolic tail at rest, indicating no significant coarctation. The graph of the right shows that after the stress induction, a diastolic tail in the descending aorta becomes clear, distal to the mildly narrowed segment, suggesting that the narrowest segment is hemodinamically significant during stress.
Conclusion:
When an athletic patient aspire to practice high-performance sports and seeks full assessment to detect potential risks, solid data must be obtained.
DSCMR could be used as a low invasive imaging method to alert, discard or confirm the appearance of future possible complications related to intense physical activity in asymptomatic well trained athletes previously dilated from AoCo looking to practice high impact sports, when a doubt raises on his hemodynamic response during stress and to make a right clinical decision is crucial.
To the best of our knowledge, this is the first case reported with this characteristics and further research is needed using DSCMR under these circumstances. More information is needed to formerly establish indications, sensibility and specificity for the proposed protocol.
Perspective:
While most of the previously dilated patients from AoCo have low to moderate exercise tolerance, those who are asymptomatic with a normal clinical and EKG evaluation at rest or with exercise, and no structural changes on TTE and/or CMR on follow up, are considered suitable for any kind of sport, except for those with large static component, according to the “Task Force 2: Congenital Heart Disease” guidelines.3,11
We present a case of a previously dilated patient from AoCo, who complained of sporadic headaches after prolonged training periods, raising concerns to his family and trainer, thus motivating the request for a written permission from the attending physician to perform sports professionally.
The difference between the blood pressure cuff and his response to exercise it may be explained by the flow-dependent gradients of coarctation. Low-level exercise can be performed in borderline cases, but will often result in the development or increase of diastolic gradient, without changing the systolic gradient.12
The evaluation of CMR at rest with close examination at the site of repair, the transverse arch, the LV size, function and LV mass was performed, but the results were not significantly abnormal, so this information was not enough for the attending physician to provide the certificate of no risk so there was no chance to just try and monitor the boy to obtain the information on to LV mass, etc.
The measurements done during exercise were not reliable, because of his quick heart rate recovery, considering the time needed to acquire Echo information at peak exercise was longer than that of the recovery.
The use of dobutamine in this scenario has not been previously described; however, it has a physiological support since it emulates the increase in cardiac output generated with exercise, exposing the significance of the coarctation during maximal exercise.
Up-to-date a complete and precise CMR protocol detects the abnormality before it becomes clinically significant, defined as peak systolic pressure gradient > 25 mmHg, with a sensitivity of 95% and specificity of 85%.13-15
After correlating the possible comorbidities in our patient, some of them related to sudden death, and the physiological adaptations of an athlete’s heart, like increase in vasovagal basal tone and compensatory left ventricular hypertrophy and dilatation, we thought that the evaluation of hemodynamic response during maximal effort would importantly contribute to give a prognostic pathway during intense physical activity. However, in the setting of an athlete’s recovery time from maximal effort equal or less than one minute, feasible evaluation of the trans-coarctation gradient on maximal effort with current recommended imaging protocols results difficult.16-19
The current Task Force 2 for congenital heart disease lacks of a recommendation regarding previously dilated patients from AoCo with borderline results on gradient tests seeking to practice high-performance sports when all other tests turned normal.3
Dobutamine was used following the standard protocol9 administered by experienced Cardiology staff and all security measures were taken.
All potential benefits and risks were fully explained to the referring physician, the patient and his relatives, whom plenty understood and formally agreed with the procedure signing a written informed consent.
It is not surprising that his velocity across the aortic arch by CMR increased when he exercised. Velocities increase everywhere when cardiac output increases, but the fact that diastolic tail becomes evident during maximal induced stress is significant.
The information obtained in this study helped the attending physician wrote a certificate of no risk for the boy so he could continue his professional sport career, since the risks to keep practicing sports in a professional manner were not enough to stop his career; there was no need to provide any specific treatment, just a long-term, close follow up, based on the following:
- He was asymptomatic except for the previously described headaches.
- His anatomy was normal as expected after a surgical repair.
- His blood pressure response to exercise was within the upper limits of normal range.
- His brachial-ankle at rest was at the limit of significance, but without upper limb hypertension and the peak gradient increased to 50 mmHg with maximal effort, with no equivalent systemic blood pressure gradient.
- There is diastolic tail in the descending aorta only during maximal stress.
Thus, in athletes previously dilated from AoCo and seeking to practice sports in a professional manner; we thought that a feasible new method capable of inducing the expected stress throughout intense and sustained physical activity allowing simultaneous and punctual measurement of variables is needed.
We found that under these specific circumstances DSCMR is a useful potential method to solve the clinical question and give the required information.
Unfortunately, there is not a threshold for re-stenosis on DSCMR since this technique has not been attempted before in this clinical scenario, however we are confident that with the right indications, in specific settings, with all security measures, it could be done to guide the clinical decision-making in borderline cases and generate the proper parameters and thresholds.
More research is needed in this field to properly clarify these observations.
References:
1. Baumgartner H: What news in the 2010 European Society of Cardiology (ESC) guidelines for the management of grown-up congenital heart disease? J Cardiovasc Med (Hagerstown) 2013, 14:100–3.
2. Secchi F, Iozzelli A, Papini GD, Aliprandi A, Di Leo G, Sardanelli F: MR imaging of aortic coarctation. Radiol Med 2009, 114:524–537.
3. Graham TP, Driscoll DJ, Gersony WM, Newburger JW, Rocchini A, Towbin JA: Task Force 2: congenital heart disease. J Am Coll Cardiol 2005, 45:1326–1333.
4. Tanous D, Benson LN, Horlick EM: Coarctation of the aorta: evaluation and management. Curr Opin Cardiol 2009, 24:509–515.
5. Vriend JW, Mulder BJ: Late complications in patients after repair of aortic coarctation: implications for management. Int J Cardiol 2005, 101:399–406.
6. Brili S, Tousoulis D, Antoniades C, Aggeli C, Roubelakis A, Papathanasiu S, Stefanadis C: Evidence of vascular dysfunction in young patients with successfully repaired coarctation of aorta. Atherosclerosis 2005, 182:97–103.
7. Stern HC, Locher D, Wallnöfer K, Weber F, Scheid KF, Emmrich P, Bühlmeyer K: Noninvasive assessment of coarctation of the aorta: comparative measurements by two-dimensional echocardiography, magnetic resonance, and angiography. Pediatr Cardiol 1991, 12:1–5.
8. Soongswang J, Nana A, Laohaprasitiporn D, Durongpisitkul K, Kangkagate C, Rochanasiri W, Kovitcharoentrakul T: Limitation of transthoracic echocardiography in the diagnosis of congenital heart diseases. J Med Assoc Thai 2000, 83 Suppl 2:S111–17.
9. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E: Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013, 15:91.
10. Tan J-L, Babu-Narayan S V, Henein MY, Mullen M, Li W: Doppler echocardiographic profile and indexes in the evaluation of aortic coarctation in patients before and after stenting. J Am Coll Cardiol 2005, 46:1045–53.
11. Buys R, Budts W, Delecluse C, Vanhees L: Exercise capacity, physical activity, and obesity in adults with repaired aortic coarctation. J Cardiovasc Nurs 2013, 28:66–73.
12. Armstrong W.F. RT: Feigenbaum’s Echocardiography. 7th edition. Lippincott Williams & Wilkins; 2012:581–582.
13. Therrien J, Thorne SA, Wright A, Kilner PJ, Somerville J: Repaired coarctation: a “cost-effective” approach to identify complications in adults. J Am Coll Cardiol 2000, 35:997–1002.
14. Van der Hulst AE, Roest AA, Westenberg JJ, Kroft LJ, de Roos A: Cardiac MRI in postoperative congenital heart disease patients. J Magn Reson Imaging 2012, 36:511–528.
15. Brown DW FD: Hurst’s the Heart. In Hurst’s Hear. Volume 2. 13th edition. Edited by Walsh RA FVFJ. New York: McGraw-Hill; 2011:1922–1948.
16. Drezner J, Berger S, Campbell R: Current controversies in the cardiovascular screening of athletes. Curr Sport Med Rep 2010, 9:86–92.
17. Bader RS, Goldberg L, Sahn DJ: Risk of sudden cardiac death in young athletes: which screening strategies are appropriate? Pediatr Clin North Am 2004, 51:1421–1441.
18. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS: Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999, 341:1351–1357.
19. Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Sugawara J, Ajisaka R, Miyauchi T: Postexercise heart rate recovery accelerates in strength-trained athletes. Med Sci Sport Exerc 2007, 39:365–370.
20. Davis A, Holloway C, Lewandowski AJ, Ntusi N, Nethononda RM, Pitcher A, Francis JM, Leeson P, Neubauer S, Rider OJ: Diameters of the normal thoracic aorta measured by cardiovascular magnetic resonance imaging; correlation with gender, body surface area and body mass index. J Cardiovasc Magn Reson 2013, 15(Suppl 1):E77.
COTW handling editor: Lilia M. Sierra-Galan, MD
Have your say: What do you think? Latest posts on this topic from the forum







