Case from: Estofan, Mariano D.; Mendez-Escalante, Jorge E.; Castro-Carvajal, Juan C.; Pons-Llado, Guillem
Institutes: Cardiac Imaging Unit, Cardiology Department, Hospital de Sant Pau, Universitat Autònoma de Barcelona, Spain
Clinical history:
A 64-year-old male, a diabetic and a smoker, with no relevant cardiovascular history presented with a syncope during sexual intercourse after using sildenafil, with complete recovery after 10 minutes.
An emergency medical team assessed the patient at home, the patient did not complain of chest pain or dyspnea, but he was found to be hypotensive (BP: 70/40 mmHg), with sinus tachycardia (120 bpm), and an oxygen saturation of 92%.
An EKG was performed.
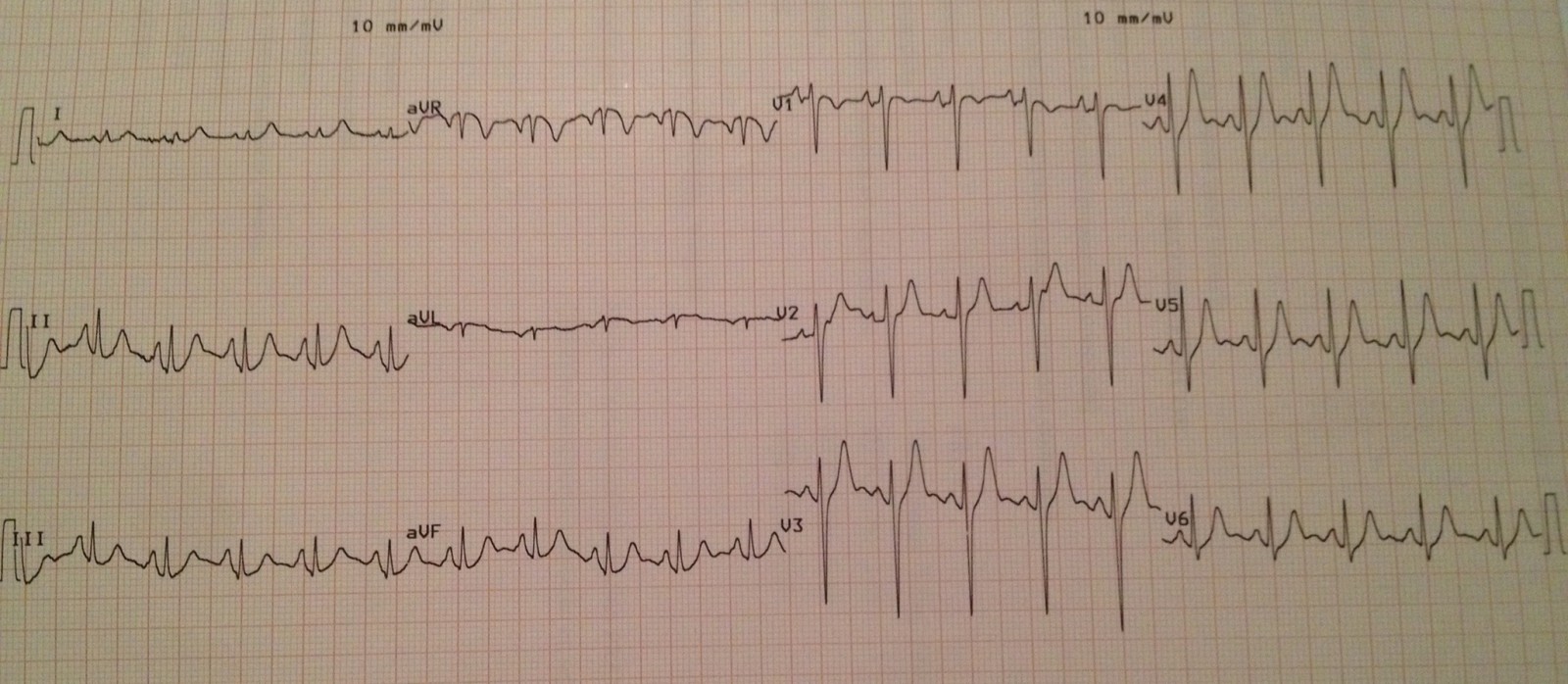
Figure 1.EKG showing no apparent signs of acute myocardial ischemia or infarction.
The patient was transferred to the hospital for further studies. An emergent MDCT scan was performed to rule out pulmonary embolism.

Figure 2a.Contrast enhanced MDCT showing pulmonary trunk and main branches appear free of any filling defect.Figure 2b.Same contrast enhanced MDC that shows evidence of pericardial effusion (circle and arrow), this finding was also confirmed at a subsequent echocardiographic study performed in the emergency department.
A second MDCT scan was performed approximately 4 hours after the first scan, this time aimed to rule out acute aortic syndrome or coronary artery disease. Although it was negative for acute aortic wall disease, coronary artery assessment in the same scan showed occlusion of the obtuse marginal branch (OM) of the left circumflex artery, with hypoperfusion of the corresponding myocardial region. Importantly, a loss in continuity of the signal of the pericardial fat was also seen, which together with the mentioned pericardial effusion, suggested localized myocardial rupture in the territory of the OM artery.
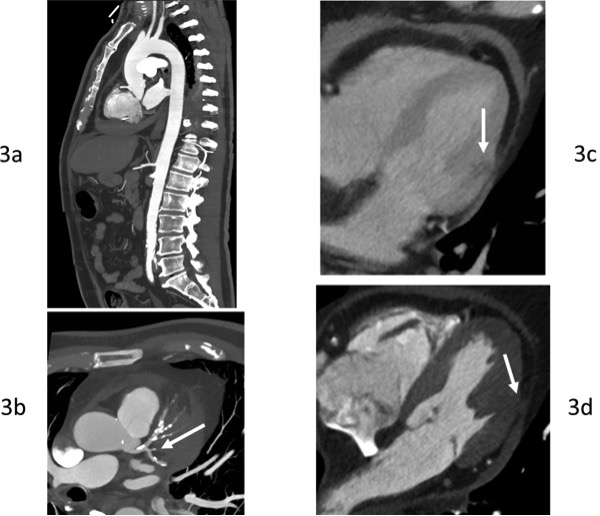
Figure 3.MDCT of aorta and coronary arteries.Figure 3a.Sagital view of the aorta.Figure 3b.Left coronary arteries assessment in the same CT scan (Left main and proximal LAD and LCX) showing the occlusion of the OM branch of the left circumflex artery(arrow). LV lateral wall showingloss in continuity of the signal of the pericardial fat (arrow).
An increase in myocardial necrotic markers was detected (hs-cTnT: 250 ng/L).
After clinical recovery, a CMR study was requested with the presumptive diagnosis of cardiac rupture.
CMR findings
SSFP short-axis cines showed mid-anterolateral hypokynesia, with a left ventricular ejection fraction of 52%, and pericardial effusion with heterogeneous signal intensity.
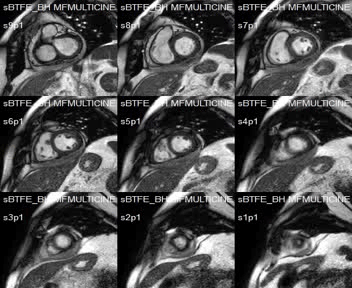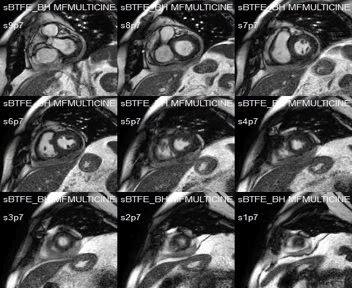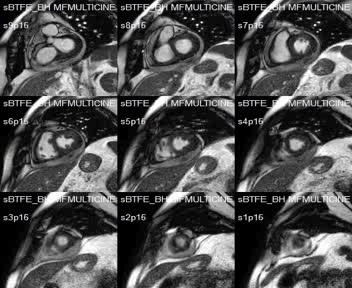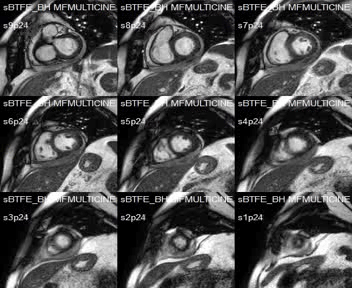
Movie 1:SSFP short-axis cines showing mid-anterolateral hypokynesia and pericardial effusion.
T2-weighted STIR sequences showed myocardial hyperintensity localized at the anterolateral basal and mid segments.
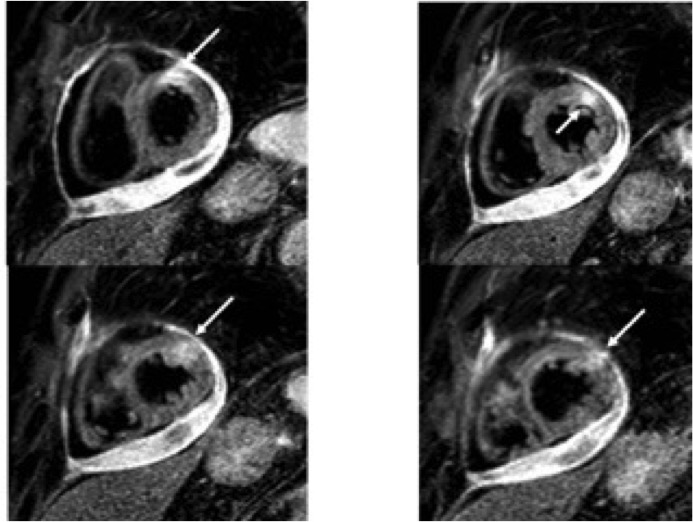
Figure 4:T2-weighted STIR short axis-views with increase signal intensity in the anterolateral segments (arrow) and pericardial effusion.
Delayed contrast-enhanced sequences showed transmural enhancement at these regions, with an area of low signal intensity in its core, bulging into the pericardial space, consistent with an intramural clot.
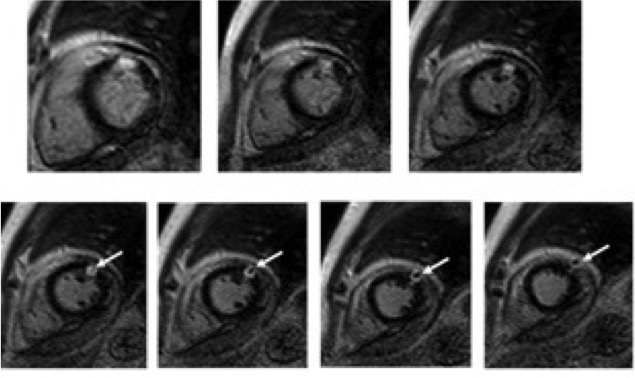
Figure 5: Delayed contrast-enhanced short axis-views with transmural enhancement in the anterolateral segments with an area of low signal intensity in its core, bulging into the pericardial space, consistent with an intramyocardial clot (arrow) and pericardial effusion.
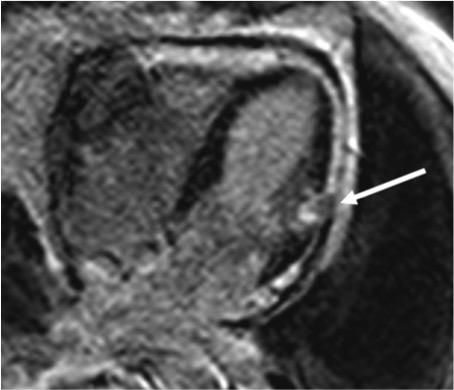
Figure 6:Delayed contrast-enhanced long-axis in 4-chamber viewwith transmural enhancement in the anterolateral segmentswith an area of low signal intensity in its core, bulging into the pericardial space, consistent with an intramyocardial clot(arrow) and pericardial effusion.
Findings were interpreted as due to an acute myocardial infarction (AMI) in the territory of the Left Circumflex artery, of reduced extension but with a subsequent contained cardiac rupture.
An invasive coronary angiography confirmed the occlusion of the OM branch.
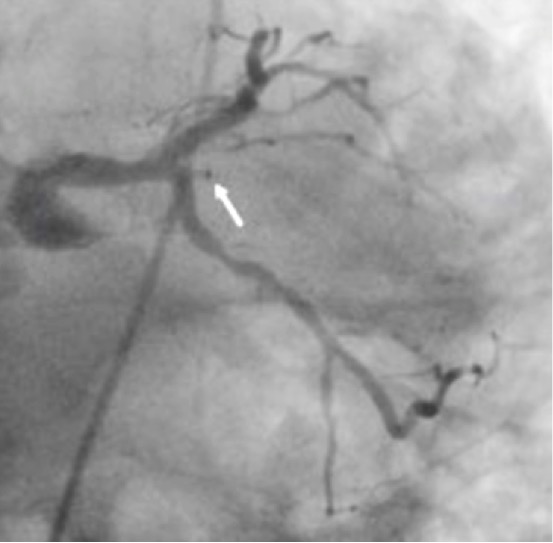
Figure 7.Invasive coronary angiography showing the OM branch occluded (arrow).
The patient was submitted to open-heart surgery, which confirmed the diagnosis; the ruptured zone was repaired by means of Teflon patch. The patient was satisfactorily discharged 9 days after surgery.
Conclusions
The diagnosis of a subacute rupture of the left ventricular free wall secondary to AMI requires a high clinical suspicion, which usually has to be confirmed by means of a multi-modality diagnostic approach. In this case, after a finding of a peculiar MDCT sign, as is the discontinuity of the pericardial fat in a region within the bed distal to an occluded coronary artery, CMR was essential to confirm the presence of AMI with contained wall rupture and, even to detect a thrombus at the site of the rupture, probably sealing an incomplete tear. Identification of an intramyocardial hypoenhanced dot within the infarcted area, suggestive of a clot, leads to a diagnosis of contained rupture.
Perspective
Rupture of the left ventricular free wall during AMI is one of the most frequent causes of sudden cardiac death. Most of the ruptures (90%) occur within 9 days of infarction, showing two peaks of incidence, within 24 hr. and between 6–9 days after AMI 1. The incidence was as high as 6% in the pre-reperfusion era, accounting for up to 30% of hospital mortality. At present, thanks to the widespread use of thrombolytic, coronary interventions, and modern therapies, the prevalence of rupture has decreased dramatically, and most of the contemporary studies, including large registries and clinical trials, report a prevalence of around 1% of AMIs, although with a mortality rate as high as 80%2. Compared with patients with AMI and no cardiac rupture, these patients are more likely to have their first AMI, usually without previous angina. Another risk factor is a single-vessel disease (usually with a totally occluded vessel), especially in combination with absence of collateral circulation, a scenario more likely resulting in transmural infarction3, like in this case.
Although acute rupture is usually fatal, some patients with a small ventricular tear, which may be temporarily sealed by clot or fibrinous pericardial adhesions, may progress to a subacute form allowing late survival. Early recognition and surgical treatment is essential for the management of these patients3. Among the diagnostic tests available, CMR is emerging as an excellent technique to clearly identify this complication, because of its high spatial resolution, and tissue characterization capacity.
Surgical results of rupture repair have varied over time with hospital mortality rates ranging from 10% to 40%. Recently, more conservative approaches, such as epicardial patch with glue without cardio-pulmonary bypass have been reported to lead to more successful outcomes4.
References:
1. Aya Takada, Kazuyuki Saito, et al. When does an infarcted heart rupture? A pathological study of 148 out-of-hospital sudden death case. Int J Cardiol 2008; 129: 447–448.
2. José López-Sendón, Enrique P. Gurfinkel, et al. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur Heart J 2010; 31: 1449–1456.
3. Sulieman Haddadin, Aldo D. Milano, et al. Surgical Treatment of Postinfarction Left Ventricular Free Wall Rupture. J Card Surg 2009; 24: 624-631.
4. Xander H.T. Wehrens, Pieter A. Doevendans. Cardiac rupture complicating myocardial infarction. Int J of Cardiol 2004; 95: 285–292.






