Case from: Paola Terlizzese1,2 MD; Vasileios Kolovos1 MD MRCP; Bharat Sidhu1 MBChB MRCP; Robert J. Huggett1 PhD MRCP.
Institute:
1) Russell’s Hall Hospital, Department of Cardiology, Dudley, West Midlands, UK.
2) Cardiovascular Diseases Section, Department of Emergency and Organ Transplantation, University of Bari, Bari, Italy.
Patient history: A 50 year old mixed race man was admitted in Coronary Care Unit after an episode of syncope and sudden onset chest pain associated with palpitations. His family history was negative for cardiovascular disease and sudden cardiac death. Furthermore his past medical history was unremarkable, except for rest dyspnea and orthopnea developed over the last 2 months.
Electrocardiogram: Showed sinus rhythm with different degrees of AV block, included periods of complete AV block and right bundle branch block (RBBB)-like escape rhythm. Frequent ventricular ectopic beats and short runs of NSVT were also seen. (Figure 1a and 1b)
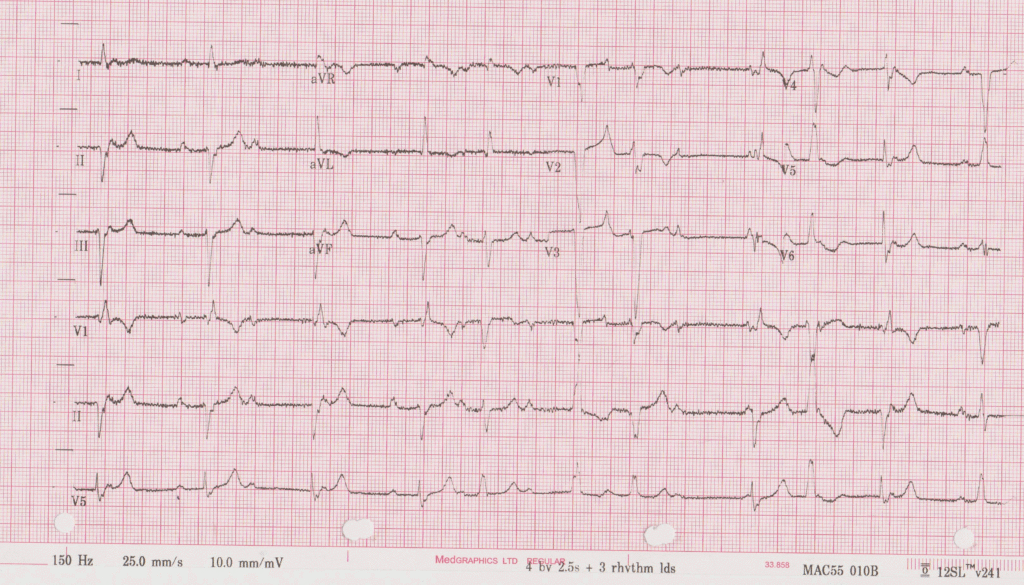
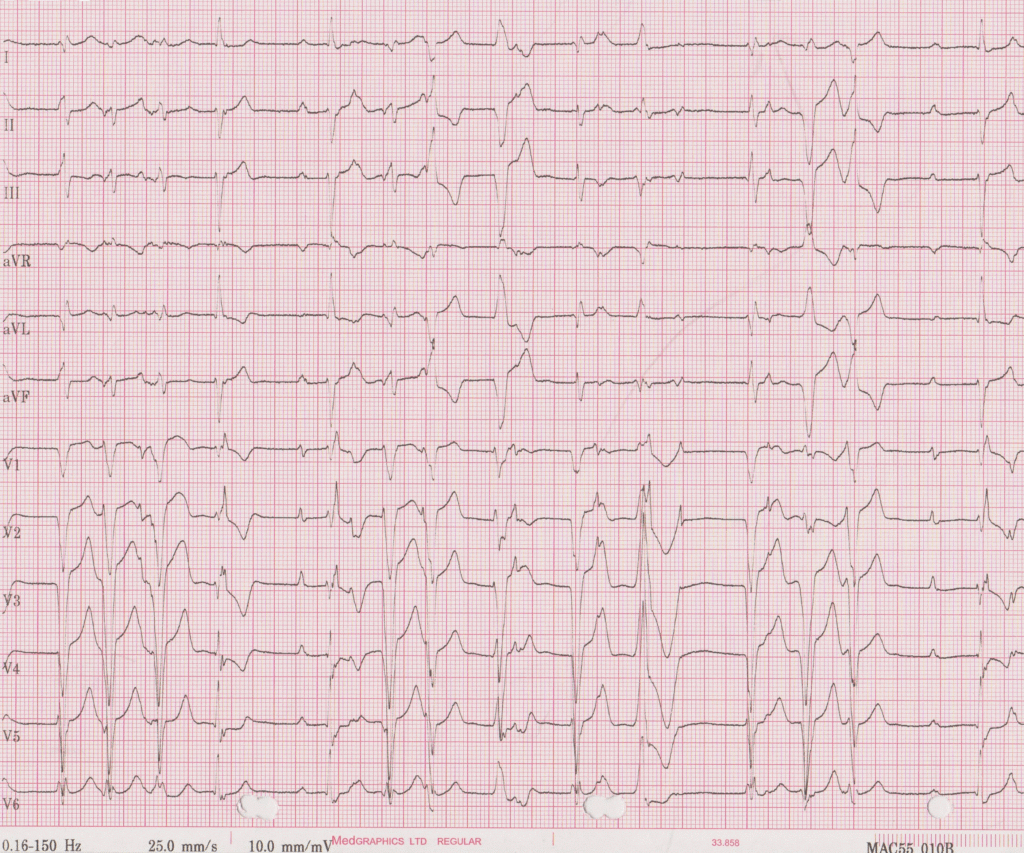
Figure 1a (top) and 1b (bottom). ECG demonstrating sinus rhythm, high grade AV block, RBBB morphology escape, frequent PVC and NSVT.
Further evaluation included:
· Blood sample: TnI was increased 92 ng/l (normal lab range 0-14ng/l); other routine-checked values including electrolytes within normal limits: CRP<3 (0-5mg/l), white cell count 6.0 x 109/l (4-11 x 109/l).
· Chest XR: within normal limits (Figure 2).
Figure 2. Chest x-ray interpreted clinically as normal.
Echocardiography: revealed severe bi-ventricular systolic dysfunction with moderate TR. Right ventricle was markedly dilated with slight improvement after diuretic therapy (Movies 1-3).
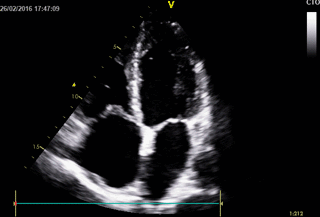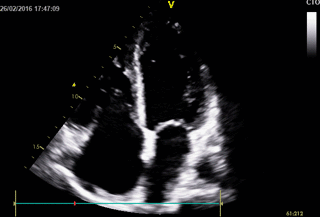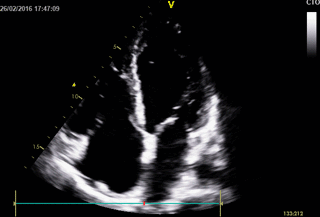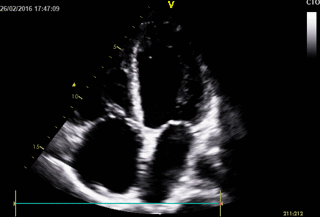
Movie 1. 2D Transthoracic echocardiogram in the 4-chamber projection showing RV and RA dilatation.
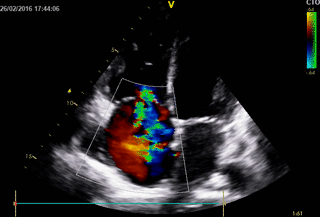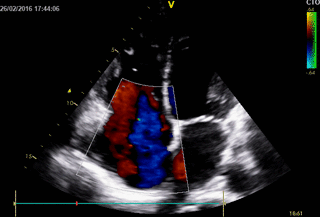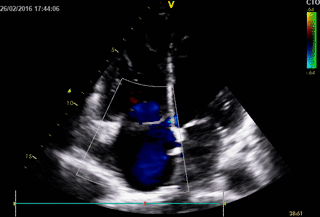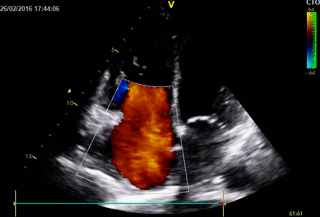
Movie 2. 2D Transthoracic echocardiogram in the 4-chamber projection with color doppler study showing severe tricuspid regurgitation.
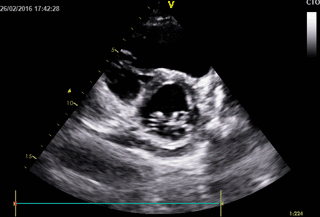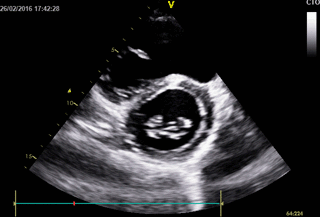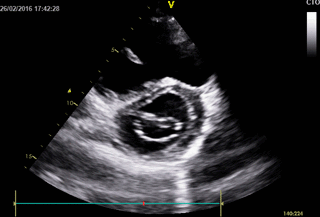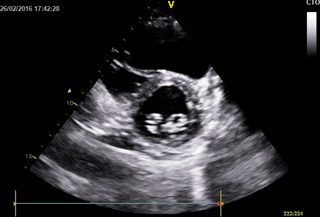
Movie 3. 2D Transthoracic echocardiogram in parasternal short axis projection showing diastolic and systolic septal flattening, signs of RV volume and pressure overload
CMR for assessment of myocardial injury: Despite initial concern related to safety issues and diagnostic quality achievable with such an irregular malignant rhythm, a CMR scan was subsequentally performed. The main aim was to further evaluate LV and RV systolic function and obtain tissue characterization through LGE study.
LV and RV were assessed with real time SSFP cine to overcome irregular rhythm issue (Movies 4-7). For the same problem T2 weighted images and perfusion study were not performed. LV internal dimensions and wall thickness were within normal limits, with dyssynchronous contraction and moderate systolic dysfunction. No regional wall motion abnormalities were noted except for basal septal hypokinesis. RV was moderately dilated with severe global hypokinesis in the presence of marked dyssynchrony. There was functional moderate TR confirmed.
LGE single-shot study revealed multifocal areas of sub-epicardial enhancement mainly in the IV septum and right ventricle (Figures 3-5). The enhancement affected the basal and mid portion at the level of inferior and superior septal hinge points (pattern consistent with involvement of AV node and proximal part of conduction system). In addition, there was RV anterior and inferior wall enhancement in an almost circumferential pattern. Small focal enhancement in the LV lateral wall were also noted.
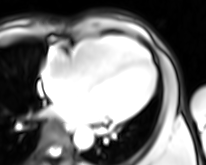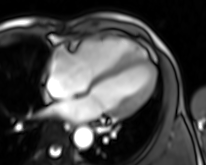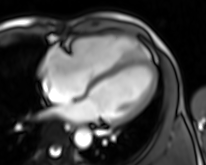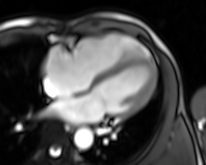
Movie 4. Four chamber real-time cine SSFP

Movie 5. Two chamber real-time cine SSFP.
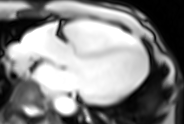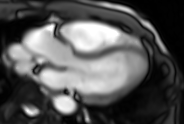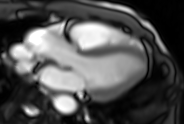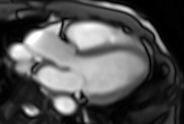
Movie 6. Three chamber real-time cine SSFP.

Movie 7. RV outflow real-time cine SSFP.
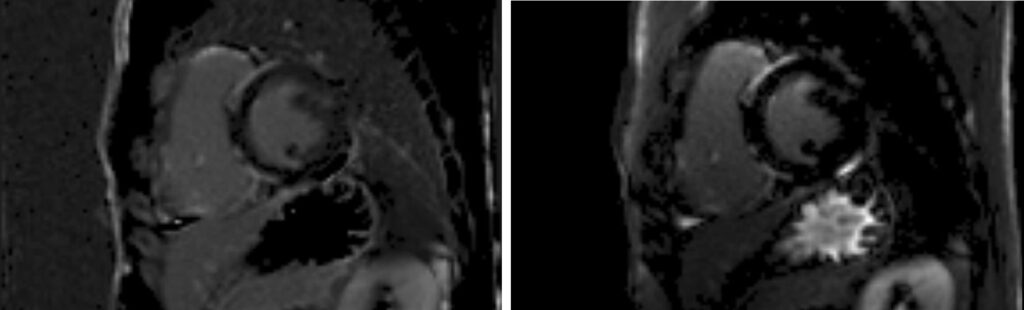
Figure 3. Late Gadolinium imaging: late-enhancement is evident in the anterior and inferior hinge points of the mid septum, on the right ventricle side with extensive involvement of inferior and anterior RV walls. Subepicardial infero-lateral LV wall enhancement can also be seen.
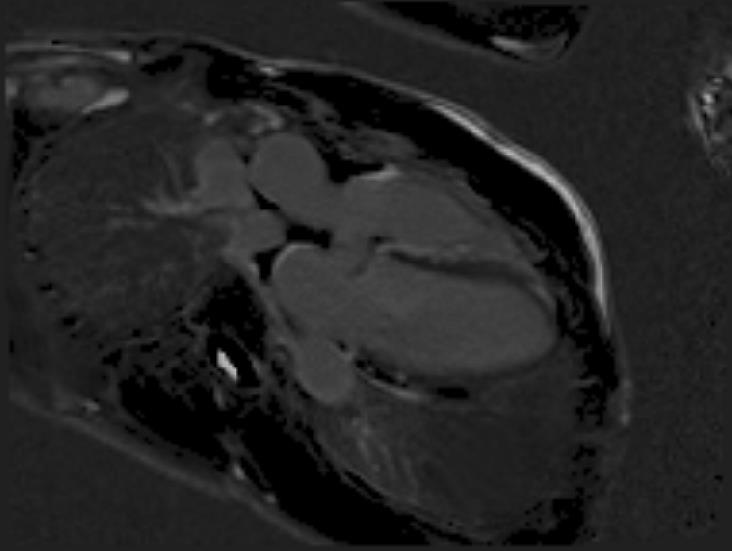
Figure 4. Late Gadolinium Imaging showing extensive septal involvement.
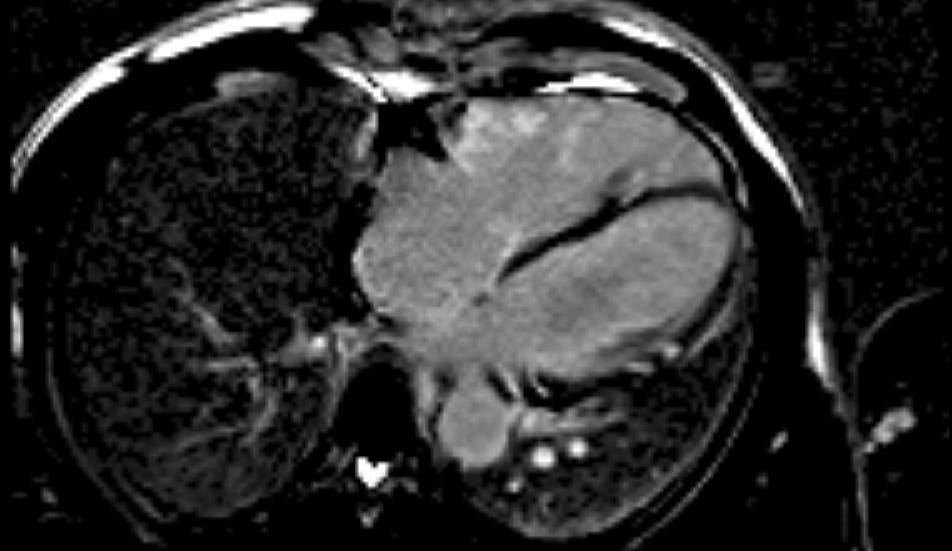
Figure 5. Late Gadolinium Imaging showing extensive right ventricular and lateral LV involvement.
These findings, together with the ECG abnormalities, we felt were suggestive of severe cardiac sarcoidosis, albeit none of the other imaging tests were pointing toward this kind of diagnosis. Therefore other tests were requested to confirm our suspicion and investigate other organs’ involvement, with the following results:
CT thorax revealed mild pulmonary interstitial disease in the upper lobes with clusters of tiny granulomas consistent with pulmonary sarcoidosis (Figure 6). Sub centimeter lymph nodes were seen at the right hilum and in the mediastinum, not suitable for biopsy.
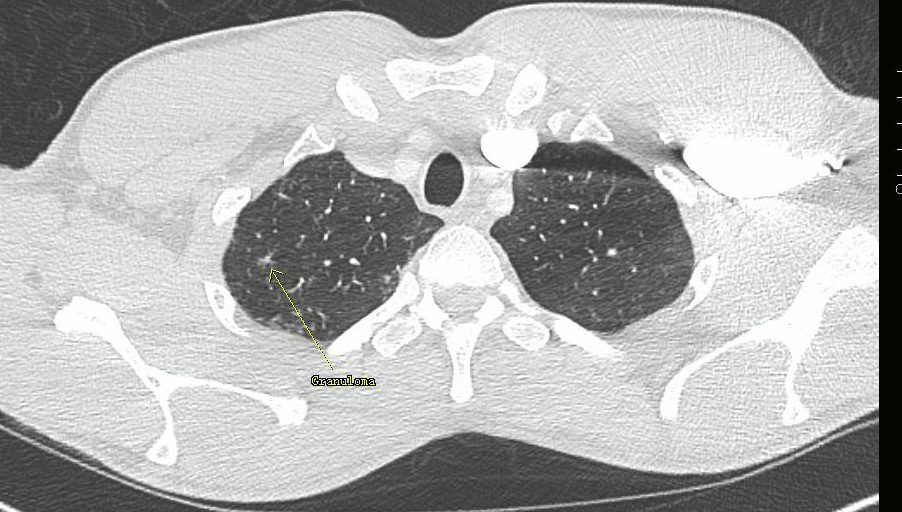
Figure 6. Transaxial CT thorax slice showing a small granuloma within the lung parenchyma.
Serum ACE levels: 91 U/l (n.r.: 5-58 U/l).
Cardiac biopsy: after a thorough evaluation of risk versus benefits of the case, it was considered not necessary as diagnosis had been already made on the basis of CMR imaging.
Therapy and Follow-up:
Severe cardiac sarcoidosis with only minor lung involvement was diagnosed and steroid therapy started with a plan to reevaluate the patient in 2-3 months. Furthermore the patient was also implanted with an ICD.1 After interval follow-up, the patient improved clinically to New York Heart Association functional class 1 and stopped all diuretic therapy. He was maintained on ACE inhibitor, beta blocker and a reducing dose of steroids. Follow-up echocardiogram however still showed moderately impaired left ventricular systolic function in the presence of paced rhythm/dyssynchrony in addition to moderately dilated right ventricle with mildly impaired systolic function. He has been referred to a sarcoid center for clinical follow-up. There has been consideration of possible bi-ventricular pacemaker although not presently a part of his device therapy.
Perspective:
Sarcoidosis is a rare disease of unknown etiology that can involve any organ within the body. Cardiac involvement in patients suffering from systemic sarcoid disease has been described in percentages variable from 5-10% (based on clinical evidences) to 27% (according to autopsy studies).2
Prompt diagnosis of myocardial involvement is of paramount importance as evidences have suggested worse outcome in patients with symptomatic cardiac involvement. Sudden cardiac death and heart failure have been shown to be the most common cause of cardiac death in several studies.3
In patients with known sarcoidosis, close monitoring over time and higher clinical suspicion may help in speeding the diagnostic path of cardiac involvement. By contrast identifying primary cardiac sarcoidosis in an otherwise healthy patient can be more challenging. In the setting of a young adult with unexplained sustained second or third AV block or new onset symptoms, in the absence of coronary artery disease or inherited forms of cardiovascular disease, CMR can be diagnostic.
In addition to the diagnostic power, LGE on CMR has been found to have a prognostic impact, showing a 9 to 30 fold higher rate of major adverse cardiac event and 11.5 fold higher rate of cardiac death as compared to LGE negative studies.4,5
Apart from stressing the importance of CMR in the diagnosis of myocardial diseases, this case wants to highlight its valuable role in the acute clinical settings in patients with unstable heart rhytm, without renouncing good quality image and diagnostic accuracy.
Furthermore this was an example of how CMR can guide therapy: showing extensive involvement of the conduction tissue and of the right ventricle, it led us on to justify the need for an ICD implantation in this patient.
Click here to view all CMR images for the case on CloudCMR.
References:
2. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid:a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204e11.
3. Ayyala US, Nair AP, Padilla ML. Cardiac sarcoidosis. Clin Chest Med 2008; 29:493.
COTW handling editor: Eddie Hulten, MD MPH FACC







