Stefania Rosmini1, Gabriella Captur1,2, Rebecca Kozor3, Heerajnarain Bulluck1,2, Anna S Herrey1, R Howard Swanton2, James C Moon1,2
(1) The Barts Heart Centre, London, UK
(2) Institute of Cardiovascular Science, University College London, London, UK
(3) Sydney Medical School, University of Sydney, Australia
Clinical History
A 55-year-old Caucasian female presented with typical angina-like symptoms. Cardiovascular examination revealed a diastolic murmur, loudest at the left sternal edge. Her past medical history included mild hypercholesterolaemia only. Electrocardiography showed sinus rhythm with no ischaemic changes.
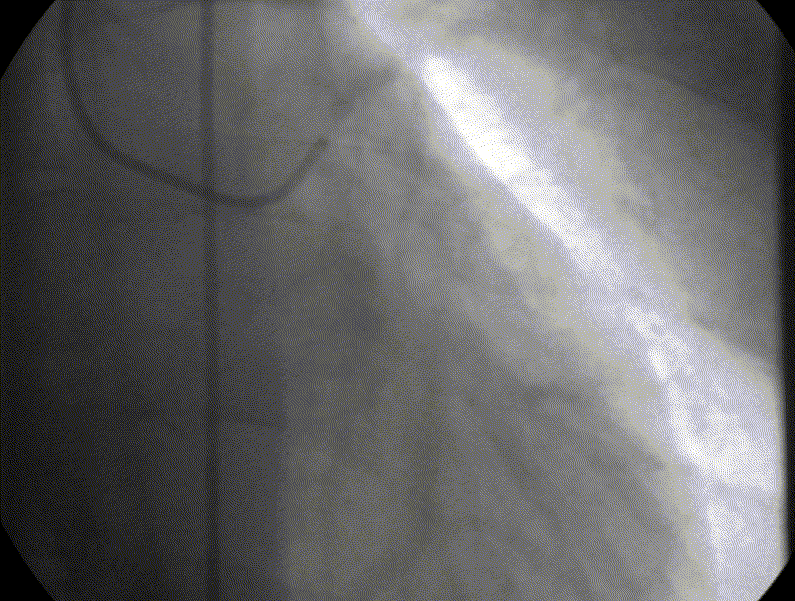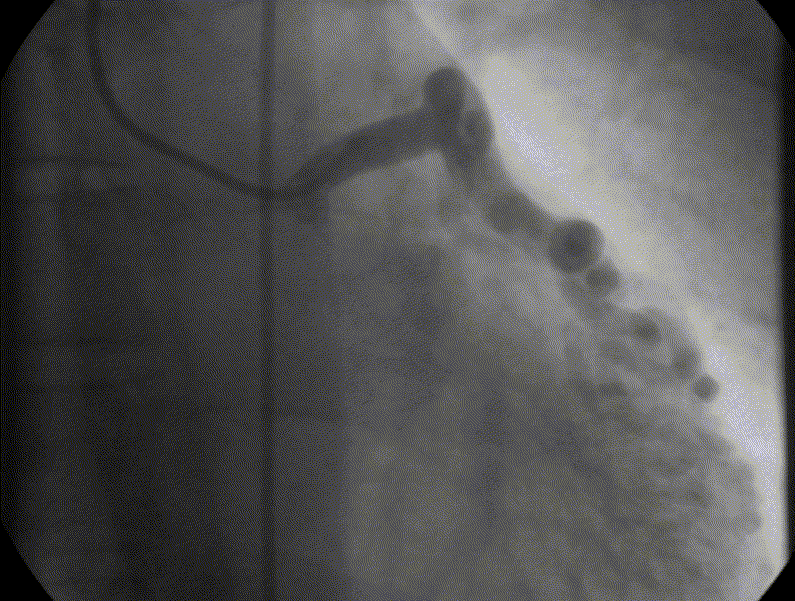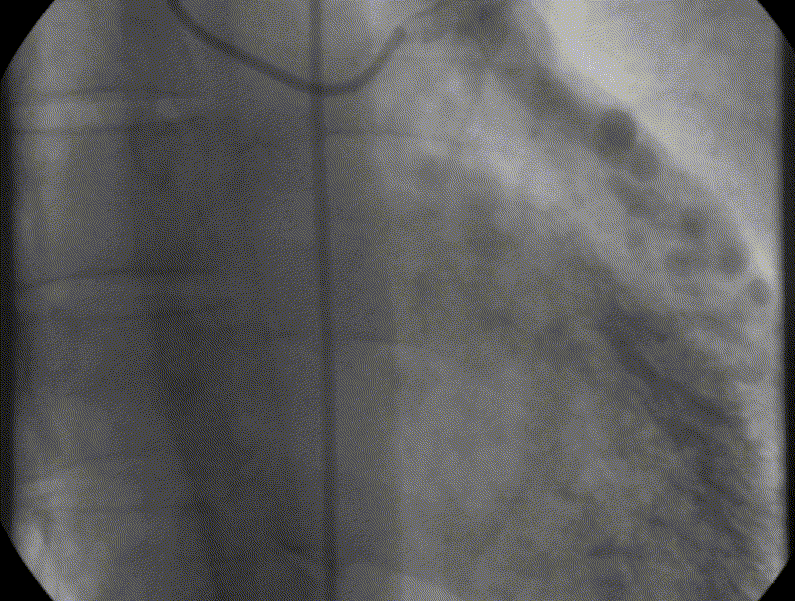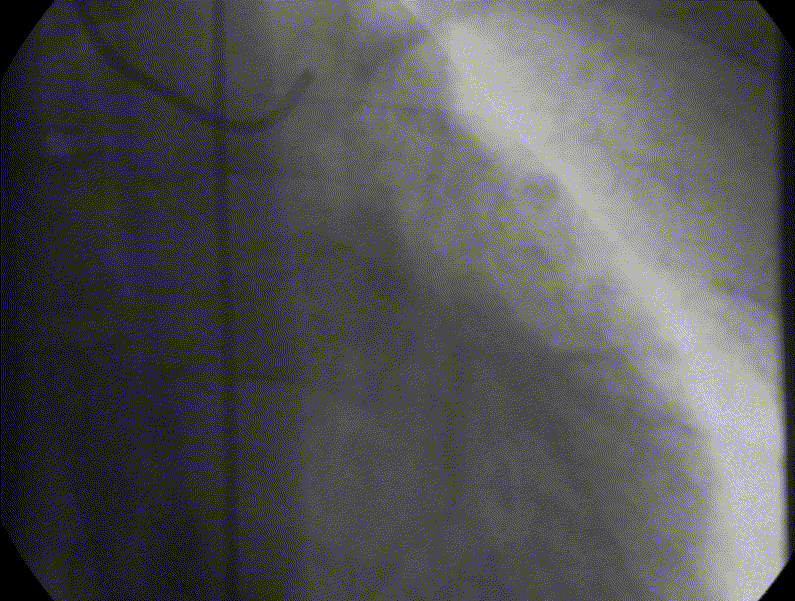
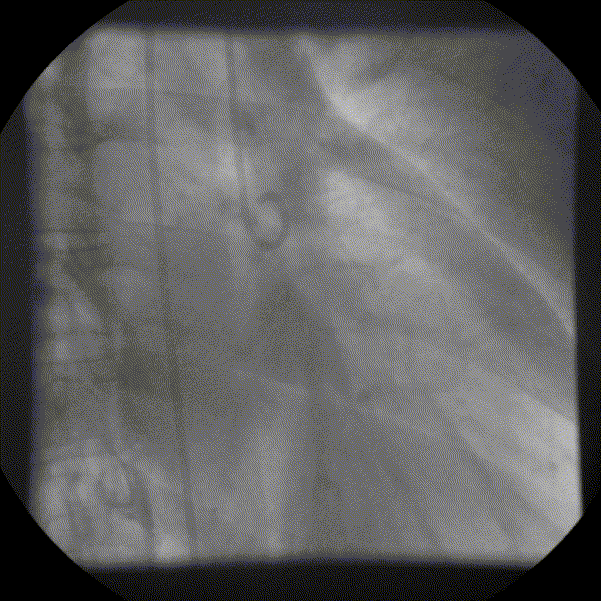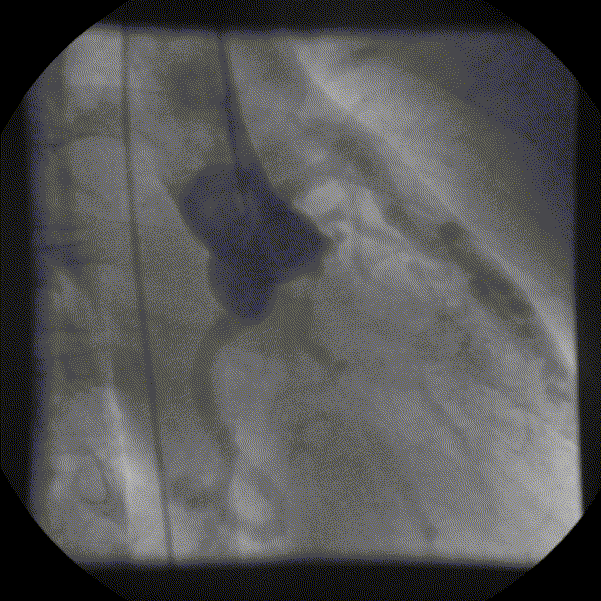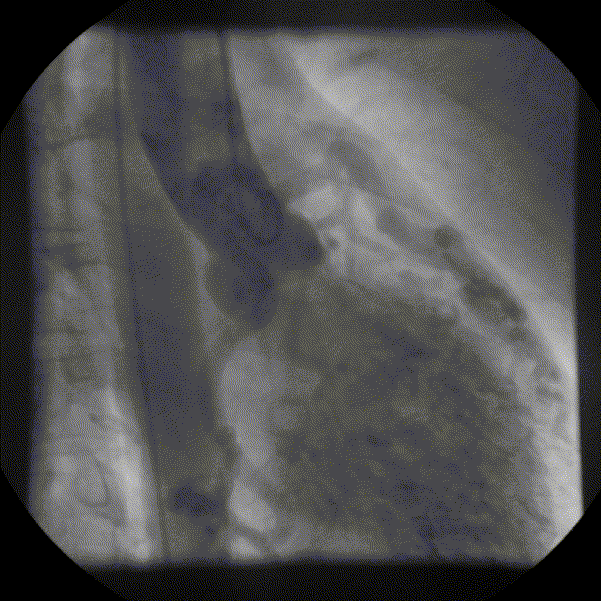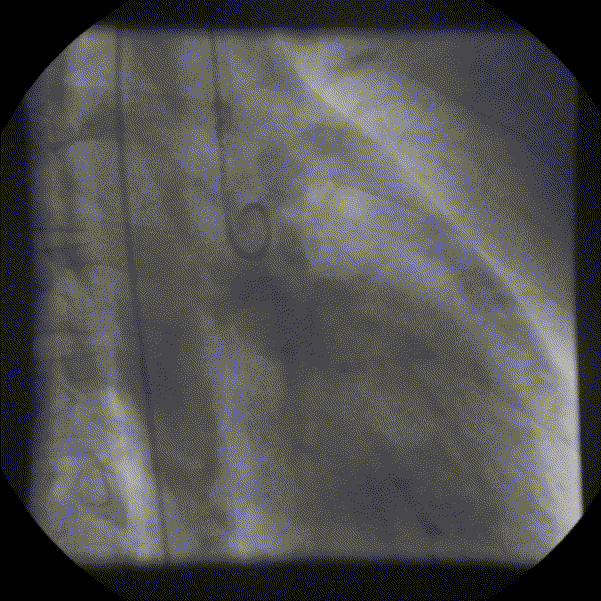
Coronary angiography showed unobstructed but markedly dilated high flow coronary arteries draining directly into the left ventricle (LV) through a plexus of small vessels arising from both coronaries (coronary-cameral communications). This mimicked aortic regurgitation on aortography (Figure 2; prompt LV opacification but not through the valve). No epicardial venous system enhanced.
Figure 1 and 2.* Coronary angiogram and aortogram showing extensive plexus of small vessels arising from both right and left coronary arteries.
*Refresh page to repeat movies in figures 1 and 2. [Ctrl+r]
CMR findings
CMR was performed to assess for inducible ischaemia using a 1.5 Tesla Avanto (Siemens Healthcare, Erlangen, Germany). LV size was at the upper limit of normal with good LV systolic function, LVEF 68%. Cine images confirmed the dilated and tortuous “corkscrew” coronaries. There was an intricate endocardial layer of small coronary-cameral communications connecting the coronary arteries and the cavities of both ventricles. A coronary sinus was present, but rudimentary. The trabecular architecture of the LV was grossly abnormal, “hazy” and hypertrabeculated.
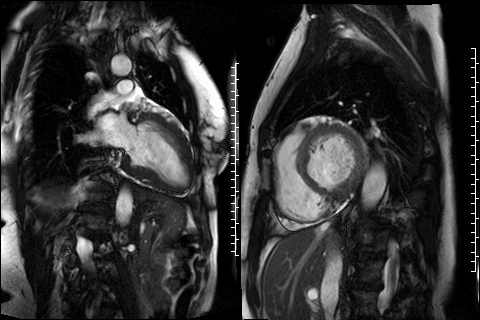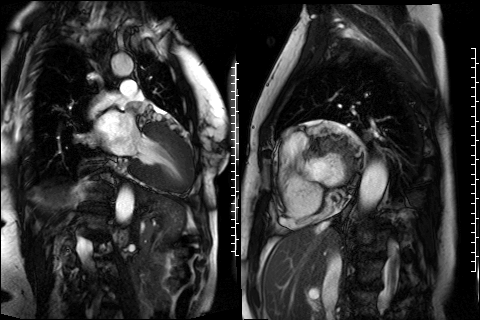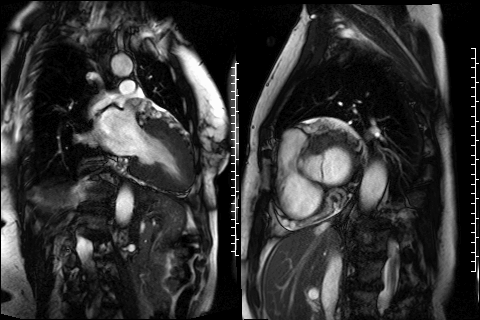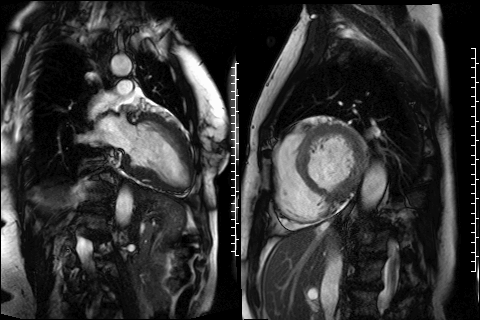
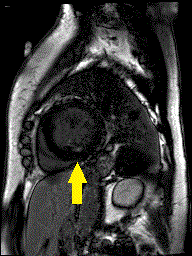
Figure 3. CMR 4CH cine showing the hypertrabeculated left ventricular architecture.
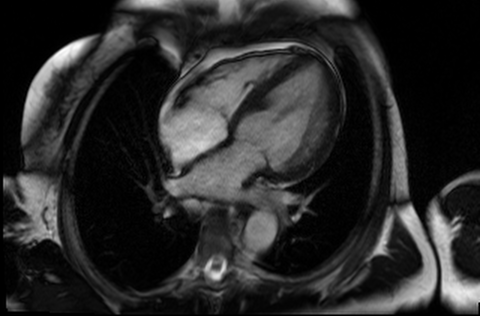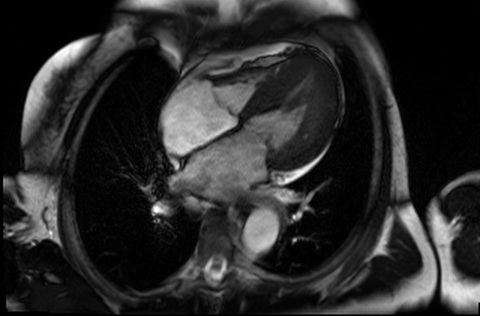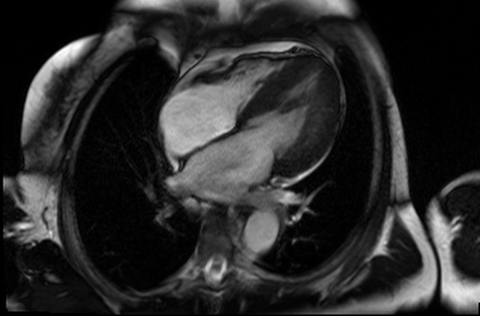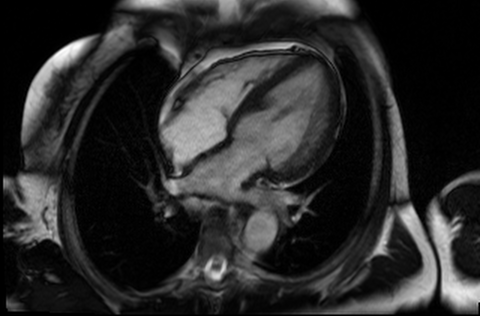
Figure 4 and 5. 2CH and basal SAX cine slices, respectively, showing the dilated and tortuous coronary arteries
Stress perfusion imaging was performed using adenosine (administered at 140ug/kg/min for 3 minutes) and showed a circumferential LV perfusion defect, most prominent in the mid inferior wall (Figure 6). Late gadolinium enhancement imaging using PSIR showed a small limited patch of subendocardial enhancement in the mid inferior LV wall (Figure 7).
Figure 6. CMR adenosine stress perfusion in the mid ventricular short axis slice, showing a circumferential inducible perfusion defect.
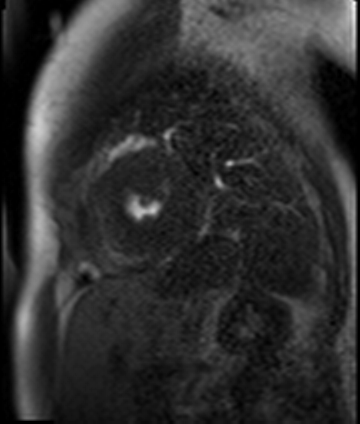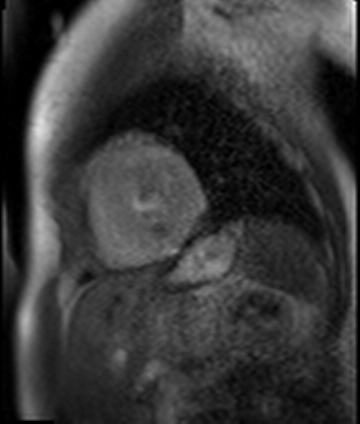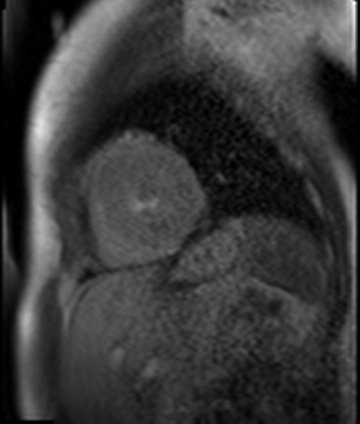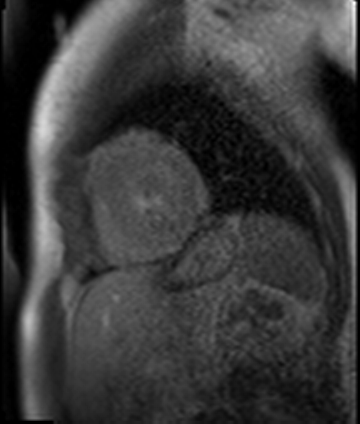
Figure 7. CMR late gadolinium enhancement image showing limited subendocardial enhancement in the mid inferior wall.
Conclusion
This case illustrates a very rare congenital malformation of the coronary drainage system including multiple coronary artery-LV fistulae. No specific management was implemented in this case – there were no known targeted surgical or medical options.
Perspective
Multiple coronary-LV fistulae with failure of the coronary venous system development is a rare congenital malformation with a reptilian heart appearance (a form of biological atavism)1 and a somewhat inefficient circulation. It has been associated with myocardial noncompaction2, probably due to a concurrent arrest in embryogenic development, leading to the persistence of embryonic myocardial sinusoids3.
The trabeculae of the left ventricle vary in health and disease and their measurement is difficult. Fractal analysis of CMR images is a mathematical solution that can measure ‘trabecular complexity’, which can help to differentiate normal from abnormal trabecular patterns. It evaluates the section-by-section fractal properties of the LV and derives a fractal dimension, which is a highly reproducible, dimensionless index of endocardial complexity, capable of detecting even subtle subclinical trabecular phenotypes. This is in comparison to the more commonly used CMR technique of noncompacted-to-compacted myocardial wall thickness ratio, which, by its nature, incorporates a measure of wall thickness that opens it up to confounders4. Fractal dimension solely evaluates the extent to which endocardial contours fill two-dimensional space, independent of the influence of wall thickness. In this case, CMR fractal analysis measured a high fractal dimension of 1.323 for the apical half of the LV (normal values for Caucasians, 1.235±0.004)5, which fits with the appearance of myocardial hypertrabeculation, and high flow through the myocardium.
There is very limited scientific data in the literature regarding this condition due to its rarity. However, it has been observed that these patients can present with angina that may culminate in myocardial infarction6. Ischemia, as demonstrated by stress CMR in this case, probably results from a steal phenomenon. The natural history and optimal management is unknown.
CMR supports the morphological diagnosis of the dilated and tortuous coronary arteries and microfistulae, the quantification of trabecular complexity, and the detection of myocardial ischaemia.
Click here to view all CMR images for the case on CloudCMR.
References
1. Walia I, Arora HS, Barker EA, Delgado RM, Frazier OH. Snake heart: a case of atavism in a human being. Texas Heart Institute journal. 2010;37(6):687-90.
2. Cartoni D, Salvini P, De Rosa R, et al. Multiple coronary artery-left ventricle microfistulae and spongy myocardium: the eagerly awaited link? Circulation. 2007;116:e81-4.
3. Black IW, Loo CK, Allan RM. Multiple coronary artery-left ventricular fistulae: clinical, angiographic, and pathologic findings. Cathet Cardiovasc Diagn. 1991;23:133-5.
4. Captur G, Zemrak F, Muthurangu V, et al. Fractal Analysis of Myocardial Trabeculations in 2547 Study Participants: Multi-Ethnic Study of Atherosclerosis. Radiology. 2015;277(3):707-715.
5. Captur G, Muthurangu V, Cook C, et al. Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson. 2013;15:36-41.
6. Hartmann M, van Es J, Galjee MA, et al. Cardiac imaging in a symptomatic patient with multiple coronary artery-left ventricular microfistulae. Heart Vessels. 2007;22:428-31.





