Sameh Khalil MD, Soha Romeih MD PhD FESC.
Radiology Department, Magdi Yacoub Foundation, Aswan Heart Center, Aswan, Egypt
Clinical history
A 59 year old male patient, diabetic, hypertensive presented with a non ST-segment elevation myocardial infarction (NSTEMI) (Image 1). The peak troponin I was 5.9 ng/ml (reference level up to 0.02 ng/ml). Coronary angiography, performed within four hours from the onset of chest pain, showed a significant proximal left anterior descending (LAD) artery lesion for which two drug eluting stents were deployed. The right coronary artery (RCA) was dominant with sluggish flow and an insignificant proximal lesion. No stents or angioplasty were performed on the RCA (Movie 1,2). ECG was recorded after catheterization (Image 2).

Image 1. Twelve lead ECG showed sinus tachycardia at 100 beat/min, normal axis, normal voltage criteria, and ST segment depression in V3-V6. Infrequent premature ventricular contractions (PVC) originating from the left ventricle (LV).

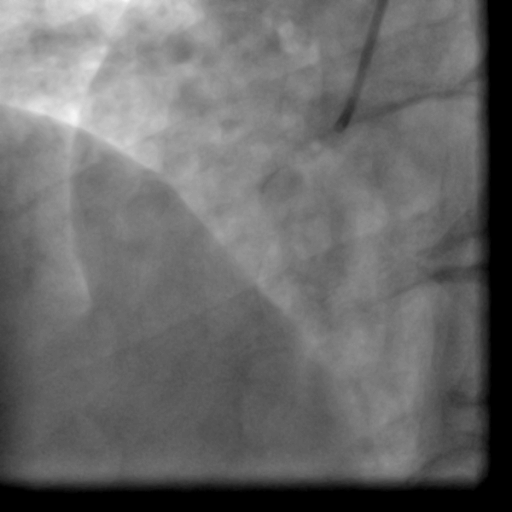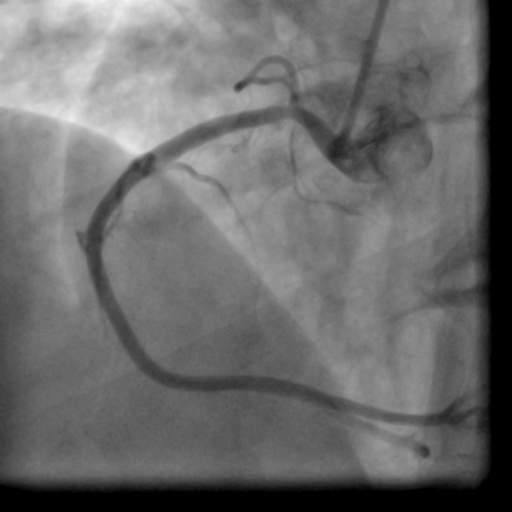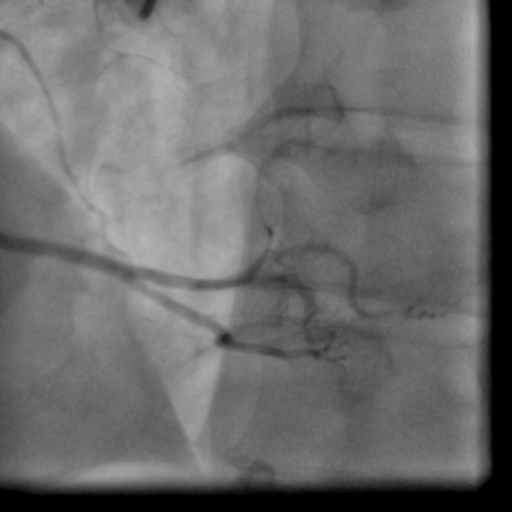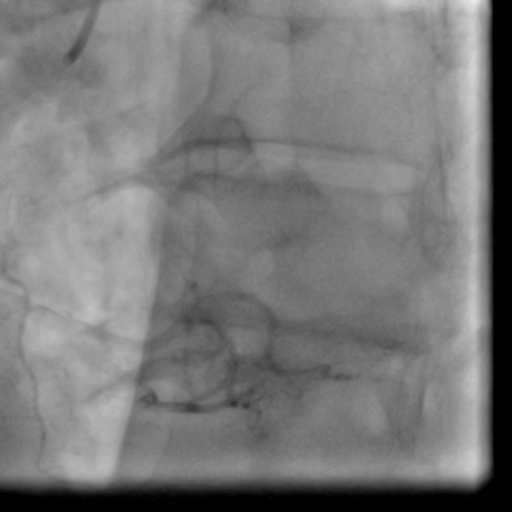
Movie 1-2. Coronary artery angiography with a selective injection in the left anterior descending (LAD) artery showed a significant proximal lesion. Selective injection in the right coronary artery (RCA) showed sluggish flow and insignificant proximal lesion.
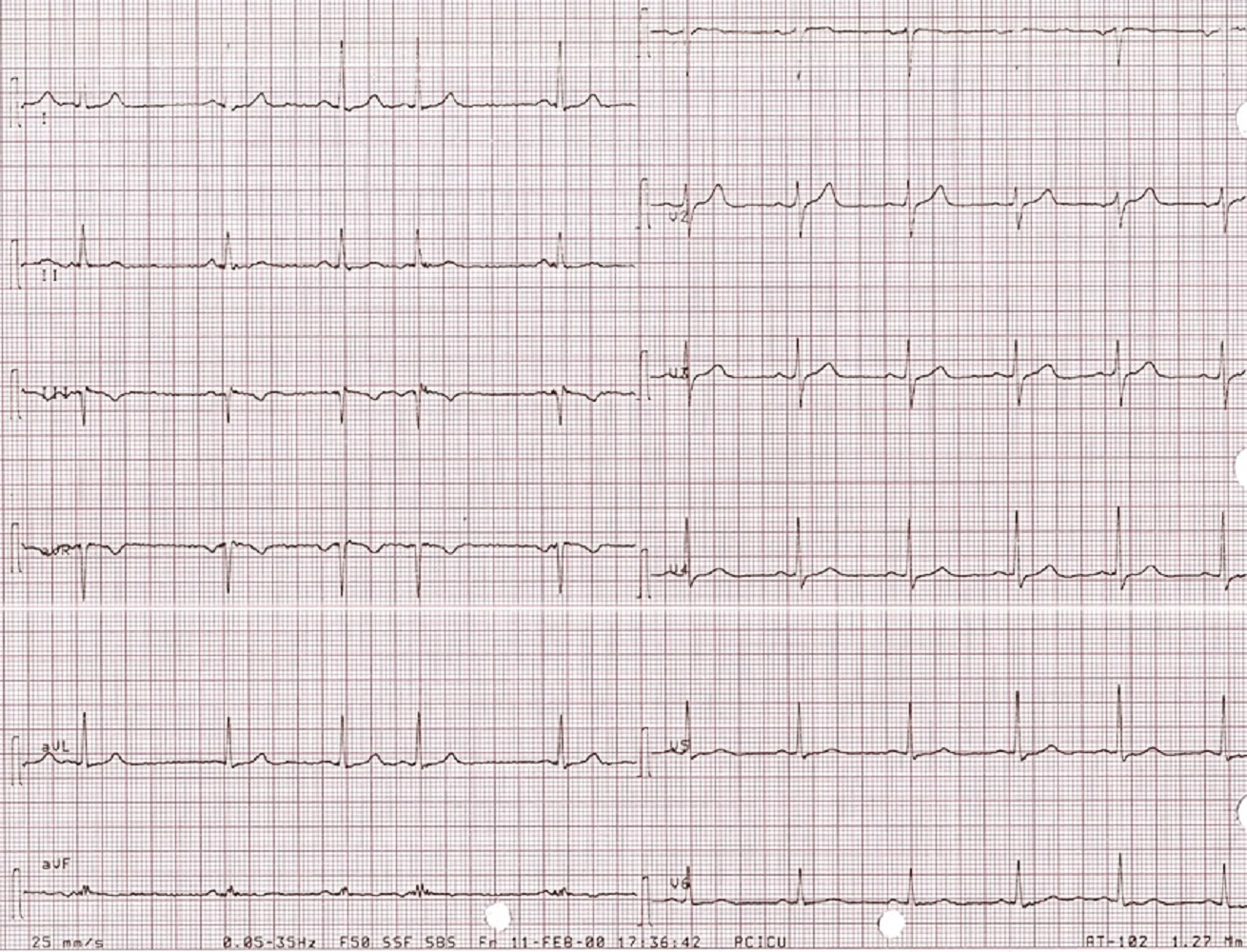
Image 2. Twelve lead ECG showed normal sinus rhythm at 60 beats/min, normal axis, normal voltage, and ST segment is back to normal in V3-V6.
Three months later, the patient presented with conscious ventricular tachycardia with left ventricular origin morphology that required DC shock (Image 3). Transthoracic echocardiography showed low-normal left ventricular systolic function (LVEF 50%) and no regional wall motion abnormalities. However, right ventricle (RV) showed hypokinesia of the RV free and inferior walls (Movie 3,4). An electrolyte profile was normal.
The patient was referred for cardiovascular magnetic resonance (CMR) to exclude underlying myocardial fibrosis related to the previous acute coronary syndrome before implantation of intra-cardiac defibrillator (ICD) implantation.

Image 3. Twelve lead rhythm strip showed ventricular tachycardia originating from the LV.

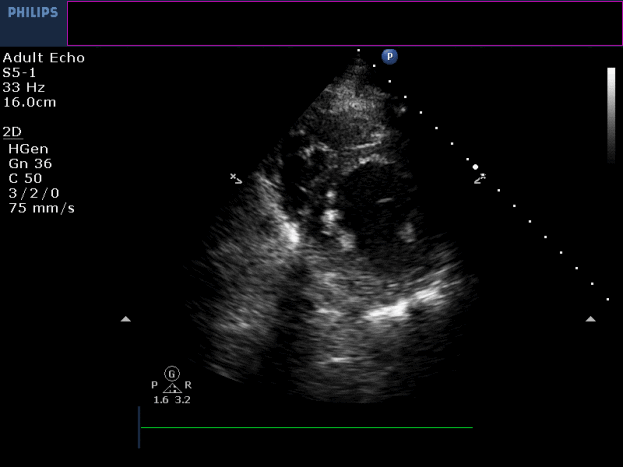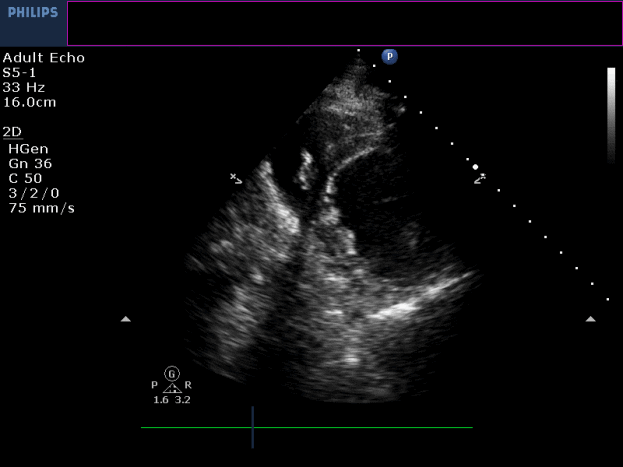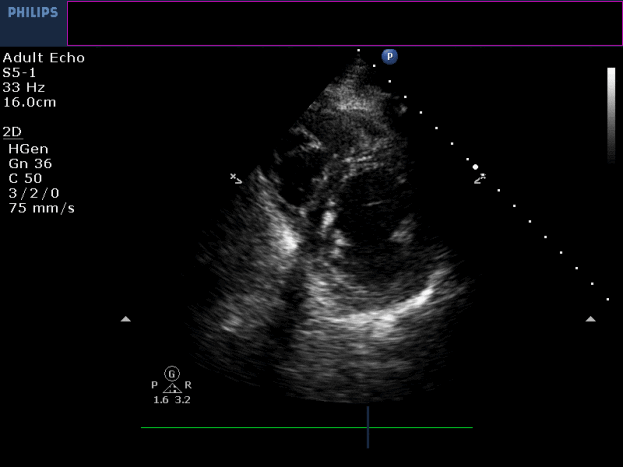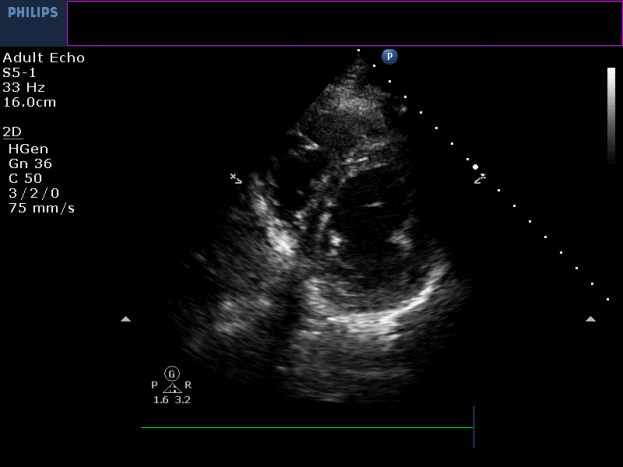
Movie 3-4. Transthoracic echocardiogram parasternal long axis and short axis view at the mid-ventricular level with hypokinesia of the RV free and inferior wall segments.
CMR Findings
CMR showed good LV systolic function (LVEF 60%) with no regional wall motion abnormalities. The RV had mild impairment of systolic function (RVEF 45%). RV volumes were normal (RV end-diastolic indexed 62 ml/m2, RV end-systolic indexed 34 ml/m2, and RV stroke volume indexed 28 ml/m2). RV free and inferior walls were akinetic (Movie 3,4) with transmural fibrosis in the corresponding segments from apical to mid-ventricular level (Image 4). LGE images showed a small focal sub-endocardial fibrosis in the inferoseptal wall at the basal level (Image 5).
The patient received an ICD. Coronary angiography performed just before ICD implantation showed well deployed patent LAD stents (Movie 5,6) and patent RCA and acute marginal branches.
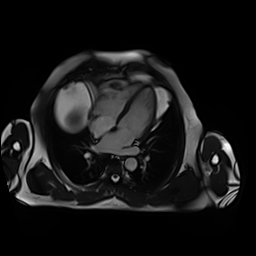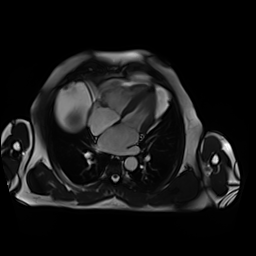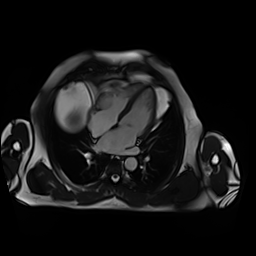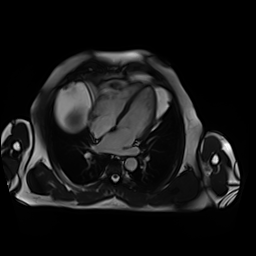
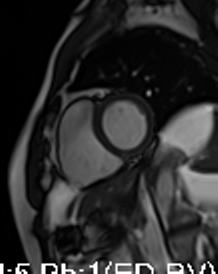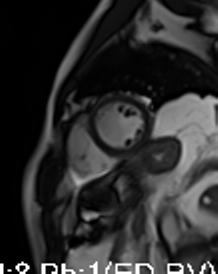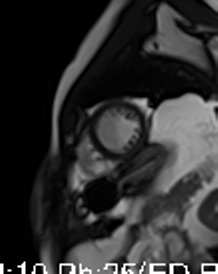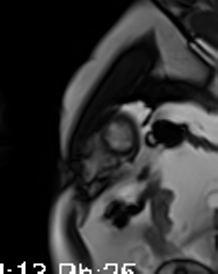
Movie 3-4. Cine four chamber and short axis SSFP shows normal left ventricular systolic function and mildly decreased right ventricular systolic function with akinetic right ventricular free and inferior walls.

Image 4. Four chamber single shot SSFP IR shows transmural late gadolinium enhancement of the right ventricular free wall.
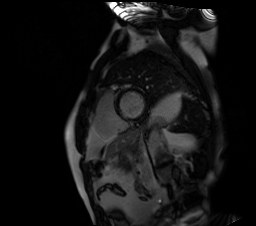
Image 5. Short axis single shot SSFP IR shows subendocardial late gadolinium enhancement (LGE) of the basal left ventricular inferoseptal wall and transmural LGE of the right ventricular free and inferior wall.
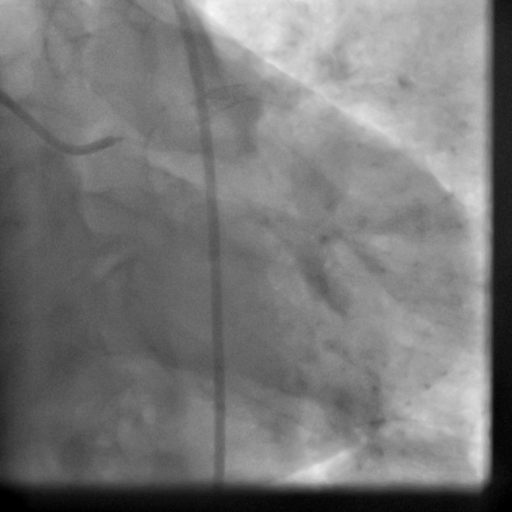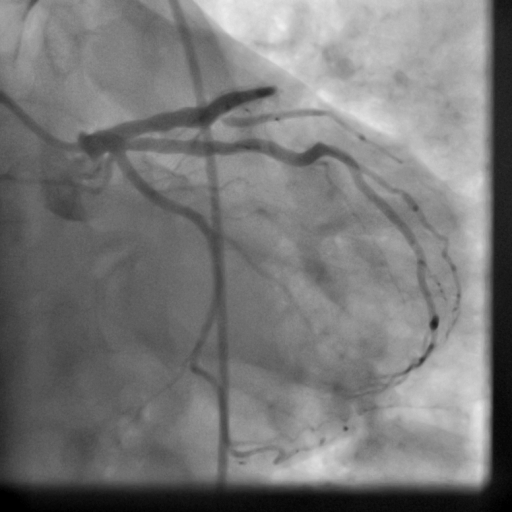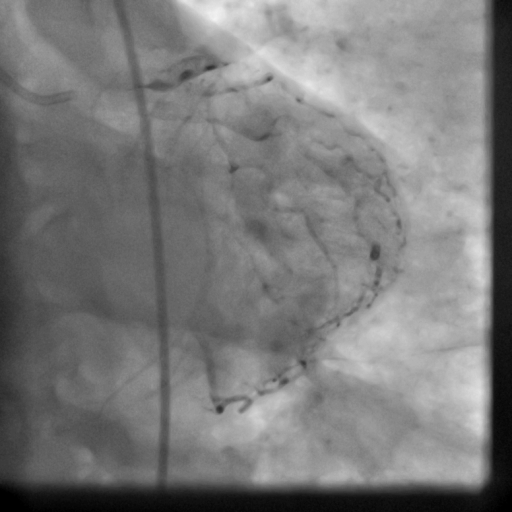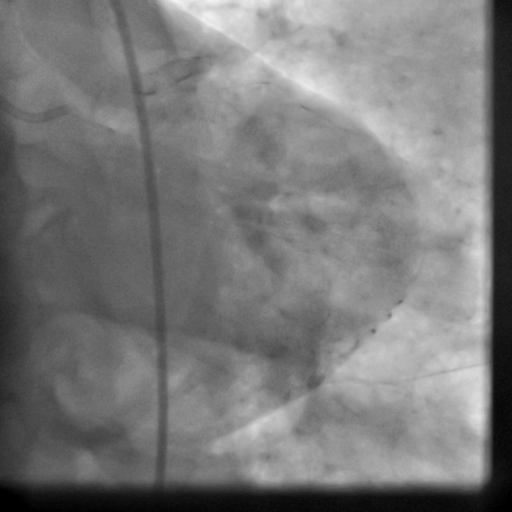
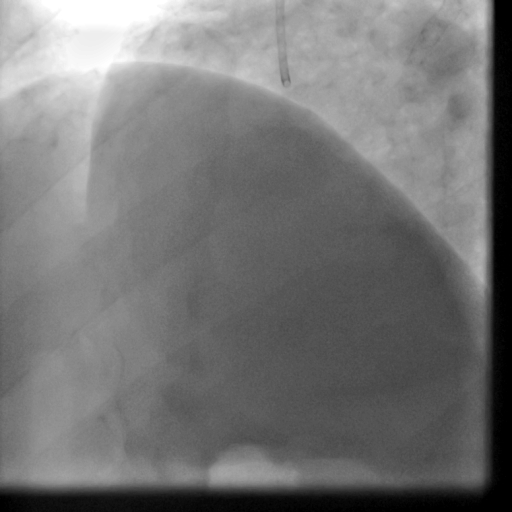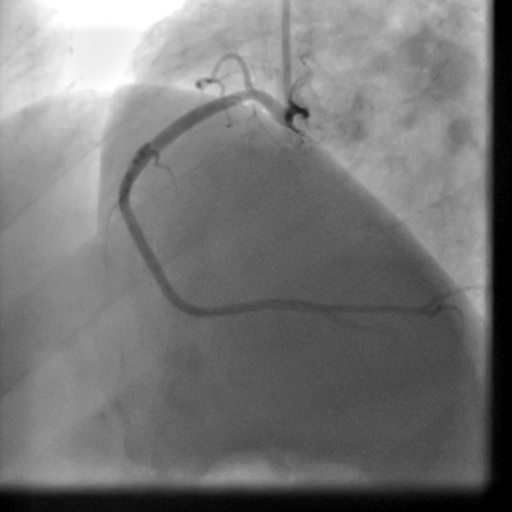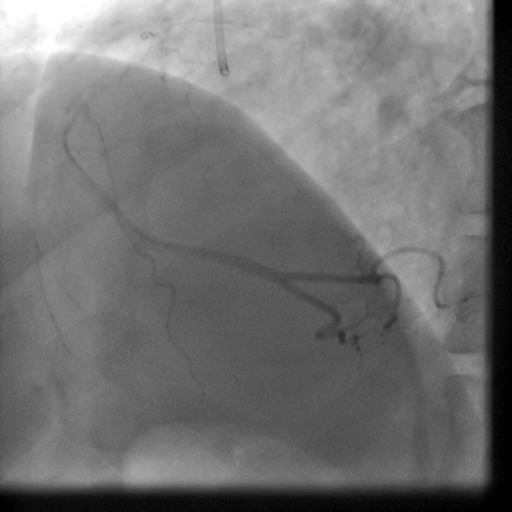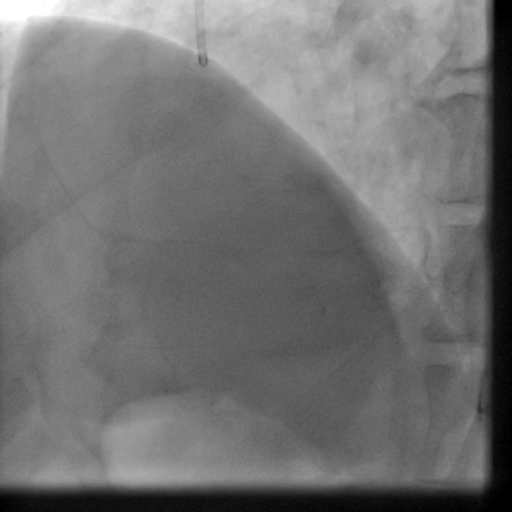
Movie 5-6. Coronary artery angiography with selective injection in the left anterior descending (LAD) artery showing a patent well deployed proximal LAD artery stents. Selective injection in the right coronary artery (RCA) shows a persistent insignificant proximal lesion, however improved flow pattern.
Conclusion
CMR is a good imaging modality to assess myocardial fibrosis. In our case, CMR revealed normal RV volumes, mild impairment of RV systolic function, and akinesia-hypokinesia with transmural fibrosis of free and inferior segments (RCA territory). These findings would suggest the diagnosis of concealed RV infarction. The RCA lesion might have been overlooked by the significant lesion in the LAD on the first coronary angiogram.
Perspective
Involvement of the RV in acute myocardial infarction has prognostic value as regard to complications and mortality. It ranges from 20-60% of inferior wall infarcts, and is rare to be isolated (1). ECG is less sensitive for RV myocardial infarction detection, and can hardly delineate RV infraction in presence of a LV infarction (2).
Detection of myocardial fibrosis pattern is fundamental for differential diagnosis of RV pathology. In our case, clinical data, ECG criteria, increased troponin level, coronary angiographic findings, and the CMR findings helped to rule out ARVC in a patient presenting with typical chest pain. None of the CMR data fit with the Task Force Criteria for ARVC diagnosis (3). Transmural fibrosis of RCA territory areas excludes the other differential diagnoses of right ventricular fibrosis as idiopathic right ventricular outflow tract tachycardia, status post myocarditis and Uhl’s anomaly (3).
LGE was first described more than 20 years ago as an excellent choice to visualize myocardial fibrosis due to its excellent endocardial visualization (4,5). Most studies with LGE have focused on the assessment of fibrosis in the LV. In 1995, the feasibility of using LGE for the assessment of RV fibrosis was introduced (6). Compared to the LV, there is a large discrepancy in the literature regarding fibrosis detection in the RV. It has been suggested that, in LGE images, accurate RV assessment needs shorter inversion time compared to the LV. In our case we successfully deliniate the viable from the scared RV myocardium using the same inversion time as the LV (7).
Free-breathing, motion-corrected, single shot LGE sequence, which was used in our study, has advantages over conventional breath hold LGE sequence especially in vulnerable patients. It has been reported that fibrosis detection and quantification are similar between free breathing, single shot LGE, and breath hold LGE when breath hold LGE can be well acquired. However, breath hold LGE quality deteriorates with a patient unable to follow breath hold instructions. Acquisition time, image quality, diagnostic confidence, and the number of successfully scanned patients are superior with single shot LGE which extends LGE based risk stratification to include patients with vulnerability confirmed by outcomes (8). Recently, free-breathing, dark blood PSIR LGE and flow independent dark blood delayed enhancement (FIDDLE) imaging have demonstrated to improve the visualization of subendocardial fibrosis in cases with low contrast with adjacent blood pool by suppression of both myocardial and blood signals (9,10).
Although our CMR was performed before ICD implantation, it is worth to mention that a wideband LGE technique eliminates the artifacts seen in LGE of patients with implanted ICDs by increasing the RF band width up to 12 kHz. The wideband LGE technique enables widespread utility of LGE images in patients with implanted cardiac devices, in whom LGE images otherwise, could not be used for diagnosis (11).
Assessment of RV fibrosis is still a controversy and exchange of multicenter experience of scan parameters could be helpful.
Click here to view case on CloudCMR
References
1. Moye S, CarneyMF, Holstege C, et al. The electrocardiogram in right ventricular myocardial infarction. American Journal of Emergency Medicine 2005; 23(6):793–9.
2. Kumar A, Abdel-Aty H, Kriedemann I, et al. Contrast-enhanced cardiovascular magnetic resonance imaging of right ventricular infarction. Journal of American College of Cardiology 2006; 48(10):1969–76.
4. Marcu CB, Nijveldt R, Beek AM, et al. Delayed contrast enhancement magnetic resonance imaging for the assessment of cardiac disease. Heart Lung Circulation 2007;16(2):70-8.
5. Wesbey GE, Higgins CB, McNamara MT, et al. Effect of gadolinium-DTPA on the magnetic relaxation times of normal and infarcted myocardium. Radiology 1984;153(1):165-9.
6. Sato H, Murakami Y, Shimada T, et al. Detection of right ventricular infarction by gadolinium DTPA-enhanced magnetic resonance imaging. European Heart Journal 1995;16(9):1195-9.
7. Grosse-Wortmann L, Macgowan CK, Vidarsson L, Yoo SJ. Late Gadolinium Enhancement of the right ventricular myocardium: Is it really different from the left? J Cardiovasc Magn Reson. 2008;10:20.
Case prepared by:
Jason Johnson, MD MHS
Associate Editor, SCMR Case of the Week
LeBonheur Children’s Hospital
University of Tennessee Health Science Center







