Kenneth E. Bujold III, DO a, Michael A. Hulse, DO b, Robert D. Tunks, MD, MHS b,c
Divisions of a Pediatric Hematology Oncology, b Pediatric Radiology, c Pediatric Cardiology, Penn State Milton S. Hershey Medical Center, Hershey, PA
Clinical History
A term male infant with a birth weight of 3,623 grams failed a routine pulse oximetry screening at approximately 36 hours of life. There were no pregnancy complications aside from maternal tobacco use. Pre- and post-ductal saturations were 94% and 86-88%, respectively. An echocardiogram was notable for a large mass in the right pulmonary artery (RPA) (Movie 1, Image 1).
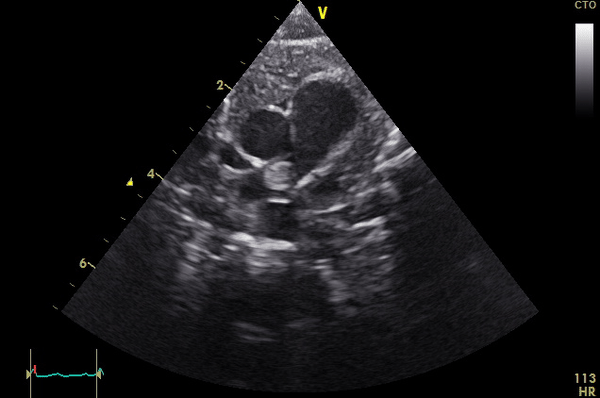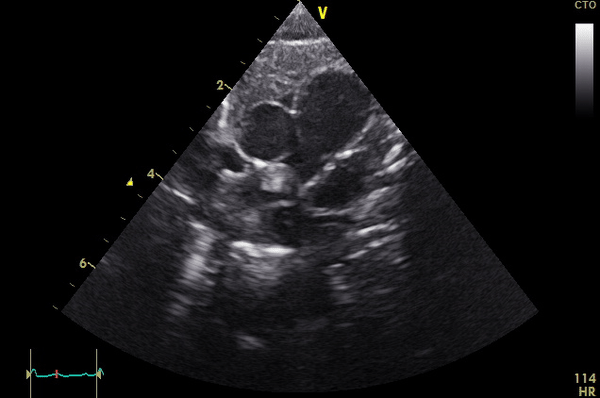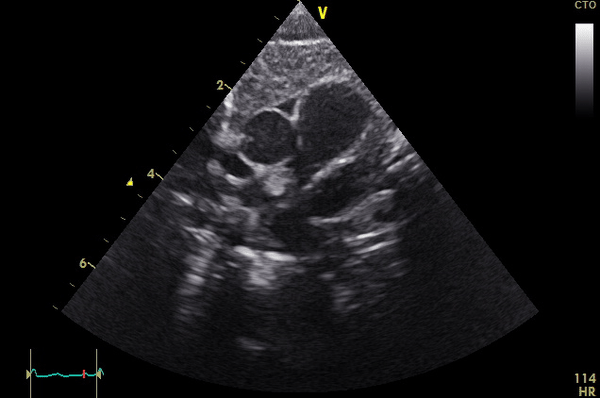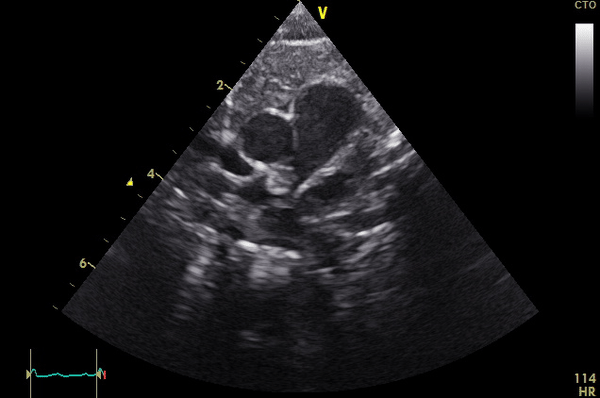
Movie 1
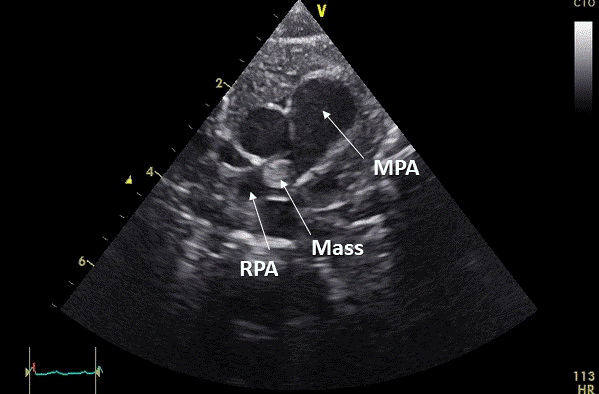
Image 1: Still frame of Movie 1. MPA: main pulmonary artery, RPA: right pulmonary artery
The heart was otherwise structurally normal aside from a patent foramen ovale with left to right shunting and a moderate patent ductus arteriosus with bidirectional shunting. The child’s hemodynamics were stable. As the infant had no known risk factors for pulmonary embolus or thrombus, cardiovascular magnetic resonance imaging was subsequently performed on a Seimens AERA 1.5 Tesla 32 channel cardiac coil magnet (Malvern, PA) to better characterize the etiology of the mass and help direct the most appropriate management. A Med-Vac system (CFI Medical, Fenton, MI) was used to immobilize the child but no other sedation or medical support was needed during the 60 minute study.
CMR Findings
Cardiac MRI performed on the second day of life delineated a large, homogenous mass in the RPA which measured 13×6 mm from an axial projection (Movie 2).
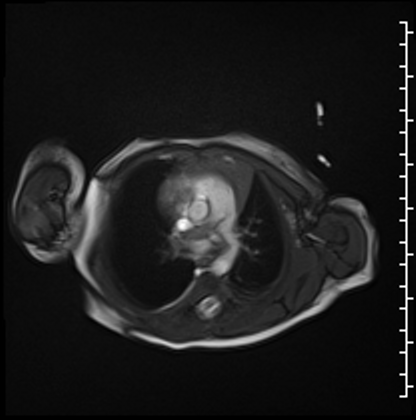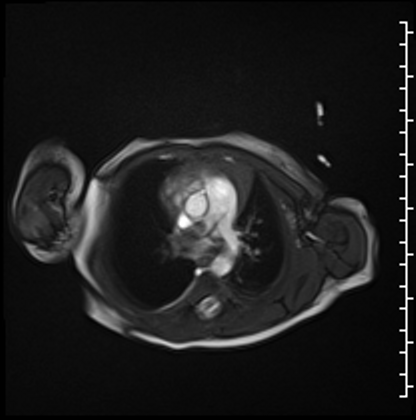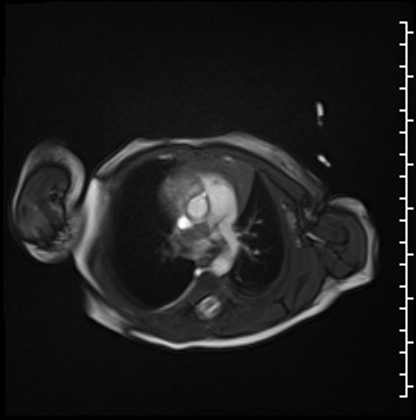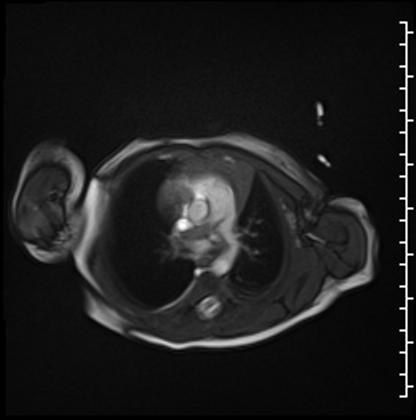
Movie 2
Right ventricular size and systolic function were normal (Movie 3).
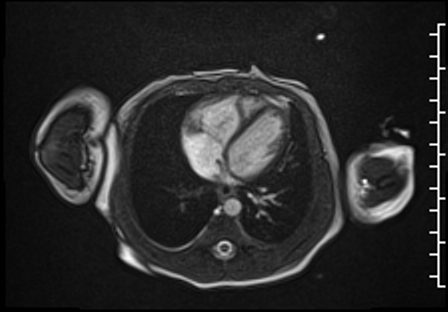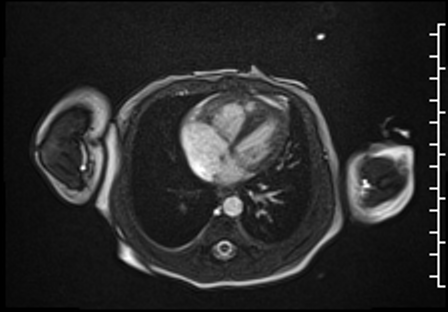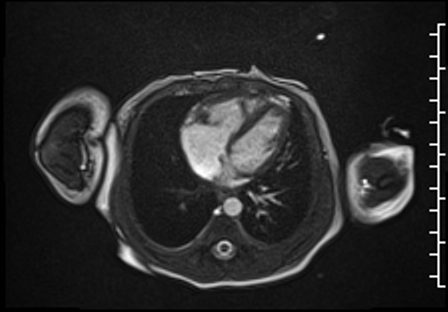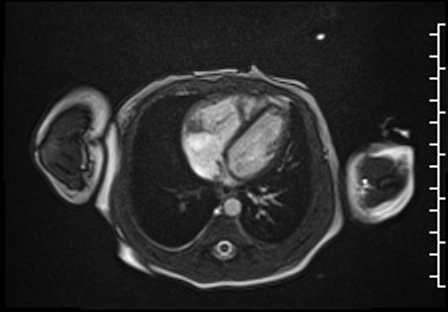
Movie 3
Right lung oligemia and only a scant amount of low velocity flow around the mass were detected (Movie 4, Image 2).
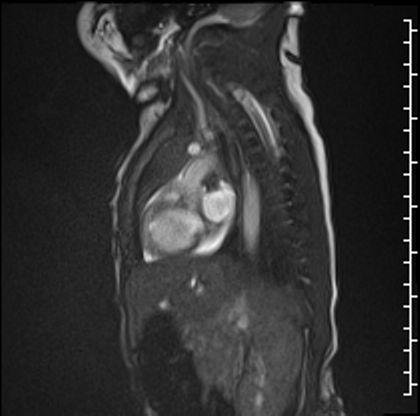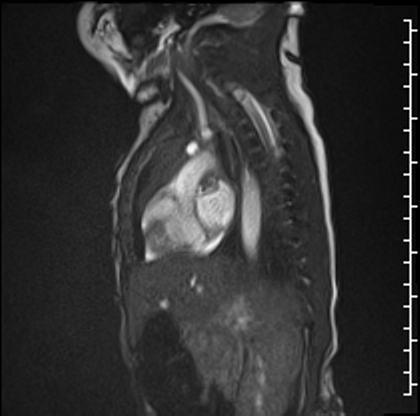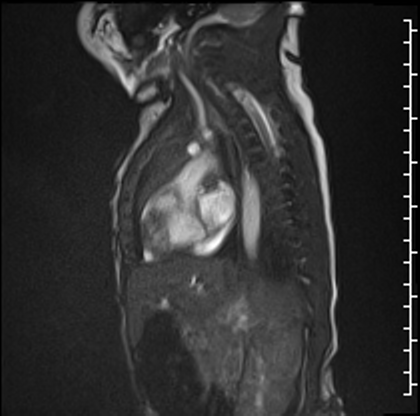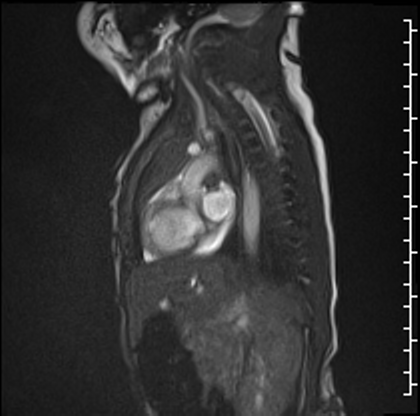
Movie 4
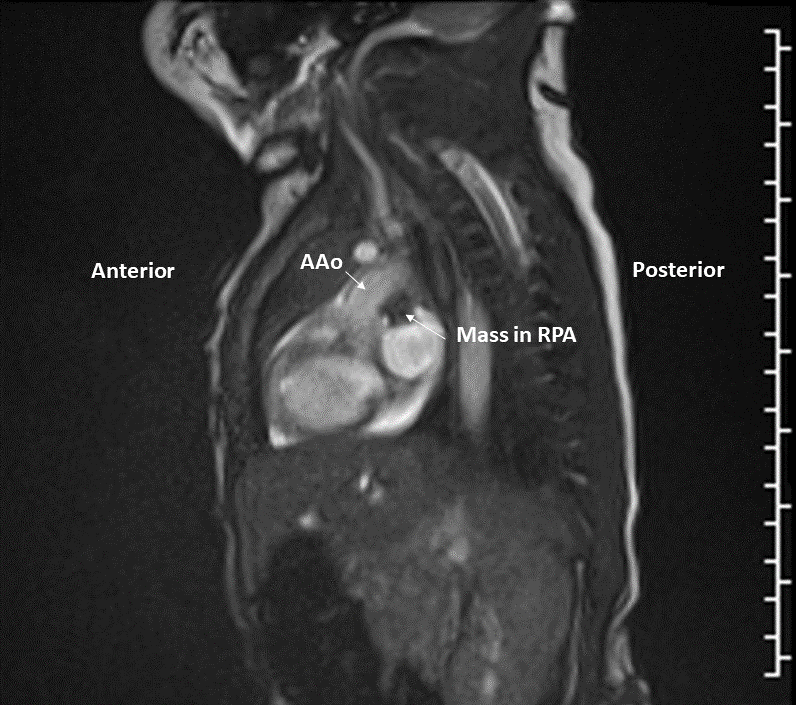
Image 2: Still frame image of Movie 4. AAo: ascending aorta)
Post-contrast high inversion time sequences obtained in an axial projection demonstrated dense signal hypointensity around the periphery of the mass, consistent with thrombus in this region (Image 3). Other etiologies of the mass could not be completely excluded as the central portion of the mass did not have a similar appearance. However, it is possible that the heterogeneity seen on these sequences is due to different stages of organization within the thrombus.
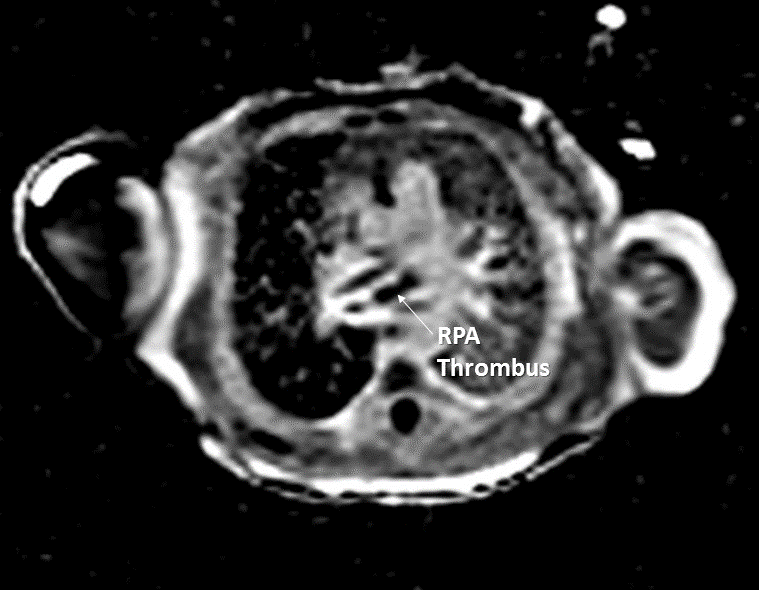
Image 3
Conclusion
Due to the extent of occlusion within the RPA, the child was started on a continuous unfractionated heparin infusion (target Anti-Xa levels of 0.3-0.7 IU/mL) after a cranial ultrasound demonstrated no evidence of hemorrhage. Prior to anticoagulation, the child underwent a limited prothrombotic evaluation which included protein C, protein S, Factor V Leiden and Prothrombin mutations, antithrombin III level and lupus anticoagulant screening, all of which were negative. On the third day of life, the child underwent surgical resection of the RPA mass which extended into the right upper and lower branches. Ligation of patent ductus arteriosus was also performed. He tolerated the procedure without complication. The mass was sent for pathology review and was consistent with thrombus.
Placental pathology was significant for velamentous cord insertion and suggestive of intervillous hemorrhage. The patient’s mother underwent a limited hypercoagulable workup that included lupus anti-coagulant screening, beta-glycoprotein antibodies, and anticardiolipin antibodies, all of which were negative.
Post-operatively, the infant was maintained on anticoagulation using unfractionated heparin. The patient remained hemodynamically stable with normal oxygen saturations. A technetium lung perfusion scan demonstrated multiple large segmental perfusion defects in both lungs involving the bilateral lower lobes, right middle lobe and lingula consistent with prior emboli. Differential pulmonary blood flow was symmetric. At last follow up, the child was thriving at eleven months of age. Follow up echocardiograms did not demonstrate any residual thrombus, right heart dysfunction or evidence of pulmonary hypertension.
Perspective
Neonatal arterial thromboses are rare occurrences and even fewer cases of pulmonary artery thrombi are reported. The majority of arterial thrombi are iatrogenic and are associated with indwelling catheters. However, we report an asymptomatic patient with no such risk factors. In addition to direct visualization of the clot, signs of a possible pulmonary artery thrombus may also include evidence of pulmonary hypertension,1,2 decreased right ventricular function,3 dilation or hypertrophy of the right ventricle,2,4 pulmonary artery hypoplasia,1 and lung hypoplasia5,6. In published case reports to date, suspected in utero thrombus development can cause lung hypoplasia5,6 and cysts.7 While the timing of the thrombolic event in our case cannot be accurately determined, one may postulate it occurred late in utero or shortly after birth as there were no other pulmonary or cardiac abnormalities detected.
To our knowledge, there are few publications describing the use of cardiac MRI/MRA as an imaging modality for a neonatal pulmonary artery thrombus.8,9 While cardiac MRI frequently requires intubation and administration of general anesthesia in neonates, our case demonstrates that sedation is not always required in high risk patients.
Click here to view the entire CMR study on Cloud CMR
References







