Clinical history
A 71 year old female was seen at the outpatient clinic with complaints of exertional dyspnea. Her medical history noted an inferior myocardial infarction 2 years earlier, for which primary percutaneous coronary intervention of the right coronary artery (RCA) was performed. This procedure had been compromised by cardiac tamponade, thought to have been caused by perforation of the RCA through wire exit. A subxyphoidal drainage was performed removing 300ml of blood and thrombi, after which patient was treated conservatively.
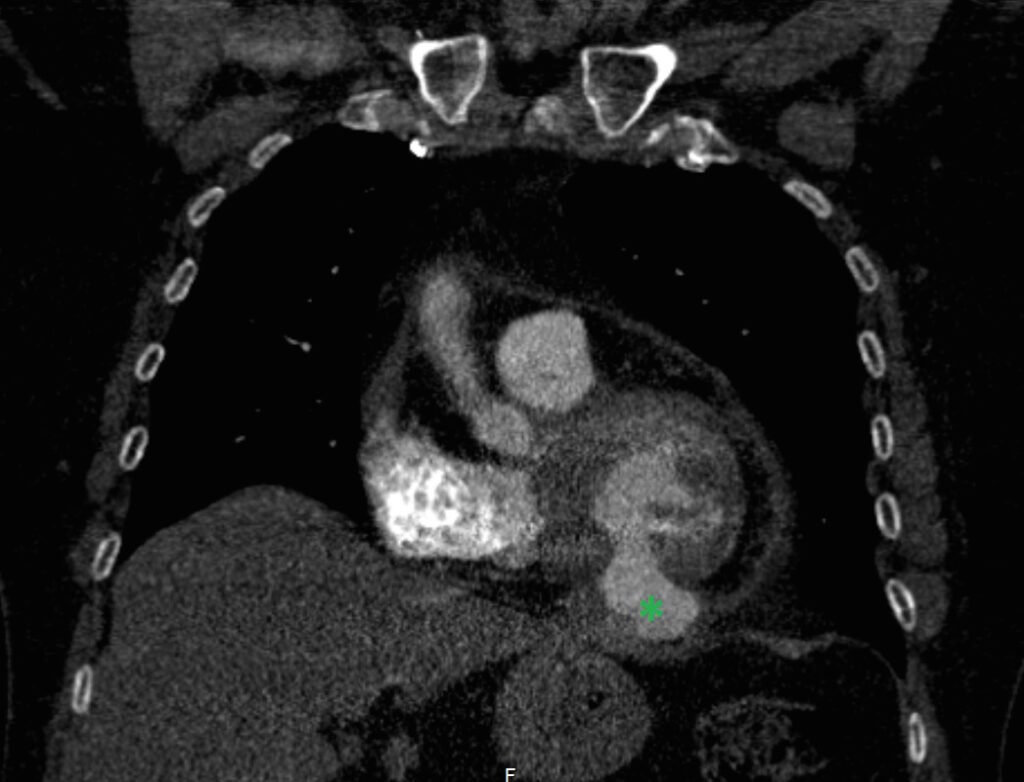
Two months after this episode, the patient presented to the emergency department with dyspnea. A CT scan was performed under suspicion of pulmonary embolism, which proved not to be the case. A left ventricular pseudoaneurysm was visible on this scan, but wasn’t reported and thus remained unrecognized.
CT findings
This study was a non-EKG triggered scan with slice thickness of 2mm. It showed no evidence of pulmonary embolism. However it does show a bulge originating from the inferior left ventricular wall (green star). The defect has a relatively small “neck” and measures 32x23mm when measured on the coronal series. There are no clear signs of thrombus formation, however due to image quality the study does not allow for reliable exclusion. This abnormality was not reported at the time the scan was performed.
CMR findings
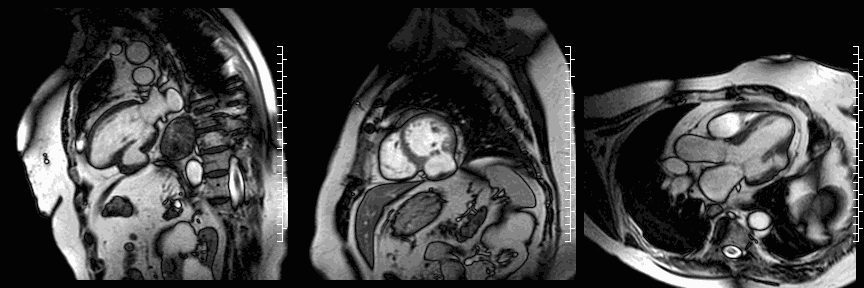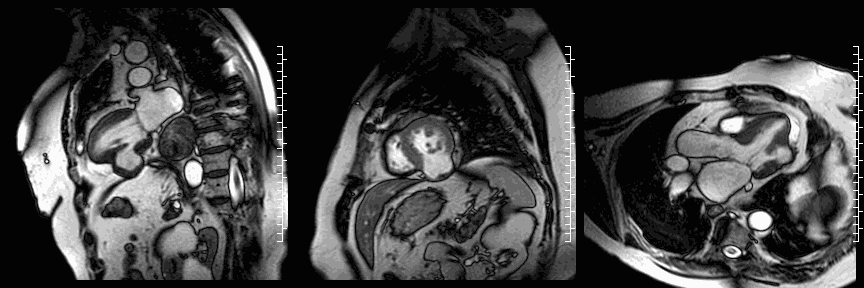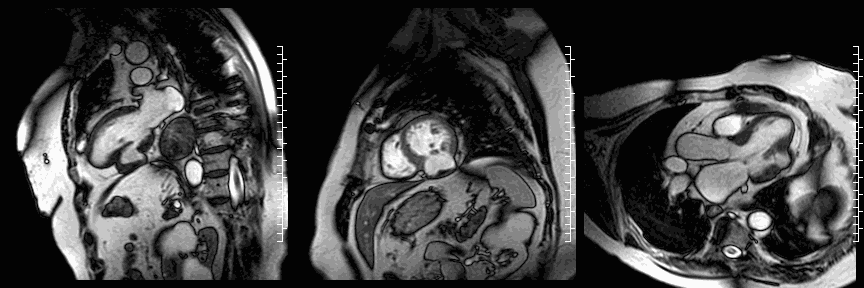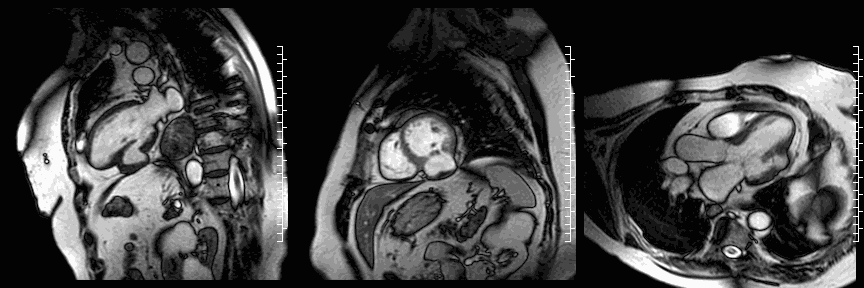
The study was performed on a 1.5T Philips Achieva MRI scanner. Cine images revealed a dyskinetic bulge of the mid-ventricular inferior LV wall, measuring 38 x 29mm. Lat gadolinium enhancement (LGE) imaging showed the defect to be thin walled, and showed a large thrombus inside. The wall itself seemed to consist of pericardium, which appeared thickened at the location of the defect. It was concluded that this was a pseudoaneurysm. When comparing the previous CT images with the current CMR it seemed that the size of the defect had not increased over time. The left ventricular systolic function was impaired due to the defect. Volumetric analysis showed a LV end diastolic volume of 164 ml, LV end systolic volume 96 ml, stroke volume 68 ml, LV ejection fraction 43%.
CMR cine images below show the dyskinetic buldge from the interior left ventricle.
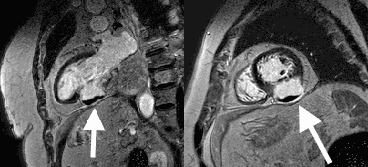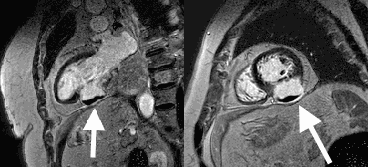
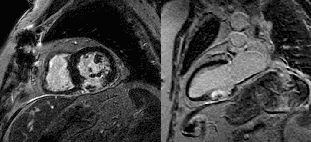
Late gadolinium enhancement (LGE) imaging below revealed the defect to be thin walled, and showed a large thrombus inside (white arrows).
Clinical history continued…
The patient was started on anticoagulation therapy. After discussion with the heart team it was decided to surgically reconstruct the left ventricle. It was argued that there was an important risk of rupture, and that the defect could increase in size over time, leading to heart failure. Furthermore, it was thought that surgery could improve the symptoms of dyspnea by improving left ventricular systolic function.
Surgical reconstruction was performed using a pericardial patch. Pathology of the material removed from the left ventricle showed organized thrombus; as was to be expected. No postoperative complications occurred. Upon visiting the outpatient clinic 2 months later, the patient was doing fine although she was still in the process of recovering. She reported slight improvement in her exercise capacity. A new CMR study was performed to assess surgical outcome and to judge whether there was still thrombus present in the left ventricle.
CMR findings (postoperative)
Cine images show the restored geometry of the left ventricle after reconstructive surgery. A very small bulge remains present. Systolic function had improved: volumetric assessment shows a LV end diastolic volume of 126 ml, LV end systolic volume 47 ml, stroke volume 79 ml, and LV ejection fraction 63%. The late enhancement series show the folded pericardial patch and suggest the presence of a small remaining thrombus.
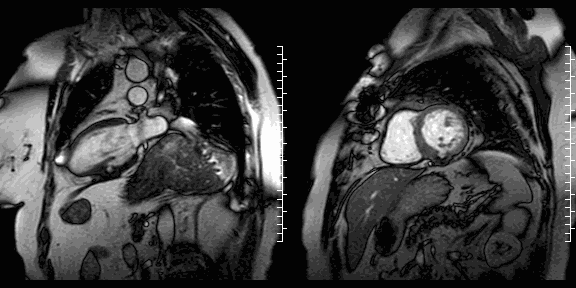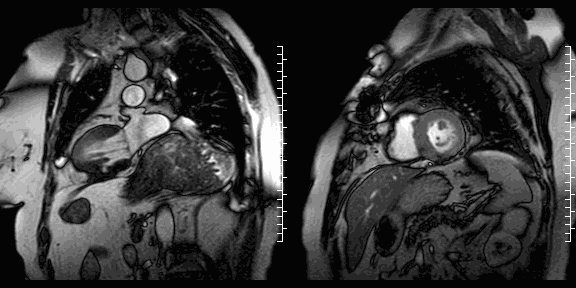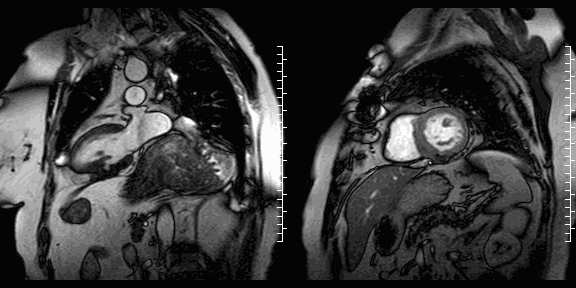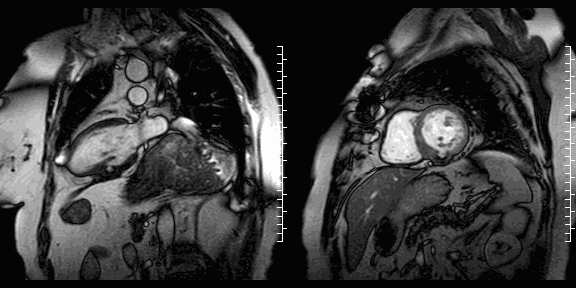
LGE images showing small remaining thrombus.
Conclusion
A 2 year old pseudoaneurysm of the inferior left ventricular wall for which successful surgical reconstruction was performed.
Perspective
Left ventricular pseudoaneurysms arise when a contained rupture of the myocardium occurs, usually after myocardial infarction. They pose a life threatening condition. The wall of a pseudoaneurysm consists of pericardial tissue, which covers the ruptured myocardium. This is unlike a true aneurysm, where the wall consists of left ventricular myocardium and scar tissue. It is generally thought that pseudoaneurysms require a more invasive treatment approach than true aneurysms, as they are more prone to rupture due their thin and unstable wall.
Consequently it is important to be able to distinguish between the two. Pseudoaneurysms originate from a rupture of the myocardium, which usually results in a more saccular appearance and a rather narrow orifice. In a true aneurysm the maximum internal width of the orifice doesn’t exceed the diameter of the aneurysm itself, where in a pseudoaneurysm it usually does. Furthermore, the presence of mural thrombus is more frequent in a pseudoaneurysm. CMR is a valuable tool to be able to distinguish these features, as our present case evidently shows. On top of this, a pseudoaneurysm can show marked late enhancement of the pericardium surrounding the defect (1). This was also present in our case.
There can be discussion about the treatment strategy chosen in this particular patient in whom the pseudoaneurysm had already been stable for 2 years. Although improving systolic function by restoring left ventricular geometry was an important consideration, the main reason for surgical correction was preventing rupture. Frances et al. described 31 patients with left ventricular pseudoaneurysm who were treated conservatively, of which 15 (48%) died at a median follow up time of less than 1 week (2). However, the remaining 16 were all alive at a median of 156 weeks. This implies that the risk of rupture is at its highest when the pseudoaneurysm has just been formed, and advocates a conservative strategy for our patient. On the contrary, there have been autopsy cases where a ruptured pseudoaneurysm of older age was found as a coincidence, implying that rupture can still occur over time (3).
In this case the CMR images provided confirmation that the abnormality seen with echocardiography was indeed a pseudoaneurysm and showed evidence of the presence of a large mural thrombus. These were valuable findings as they determined the subsequent treatment of the patient. CMR clearly was superior to echocardiography in this case and should be ordered when there is suspicion of the presence of a pseudoaneurysm.
Click here to view the entire case on CloudCMR
References
1. Konen et al. True versus false left ventricular aneurysm: differentiation with MR imaging. Radiology 2005; 236:65-70
2. Frances et al. Left ventricular pseudo aneurysm. J Am Coll Cardiol 1998; 32:557-61
3. Vlodaver et al. True and false left ventricular aneurysms. Propensity for the latter to rupture. Circulation 1975; 3:567-72
Case prepared by Associate Editor Dr Rebecca Kozor, University of Sydney, Australia





