Julia Kar PhD, Bryant M. Baldwin, Michael V. Cohen MD, Shane Joseph MD, Anam Chowdhry MD, Maria S. Figarola MD, Christopher Malozzi DO
Departments of Mechanical Engineering and Pharmacology, Departments of Medicine, Physiology and Cardiology, Department of Radiology, Department of Cardiology, University of South Alabama, Mobile, Alabama
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical History:
A 54 year old woman was diagnosed with left-sided breast cancer in October 2015. She was treated with chemotherapeutic agents (CA) Adriamycin (cumulative dose of 400 mg/m2), cyclophosphamide and trastuzumab (Herceptin®). She also received ADO–trastuzumab emtansine (Kadcyla®) for a history of metastatic breast cancer previously receiving treatment with Herceptin®. During her chemotherapy regimen an echocardiogram revealed lowered left ventricular (LV) systolic function (LVEF 48%). She was subsequently evaluated for the possibility of CA induced LV cardiomyopathy. Due to her decreased LVEF continuous measurements of LVEF accompanied the Herceptin® treatment. Prior to chemotherapy, she had a past medical history of hypertension, left bundle branch block (LBBB) and hyperlipidemia, and was on atorvastatin and carvedilol (12-lead ECG, Figure 1). At this time the CA included carboplatin, gemcitabine, and Herceptin®. Given the decreased LVEF and existence of cardiovascular comorbidities, there was an immediate need to establish a diagnosis of cardiotoxicity.
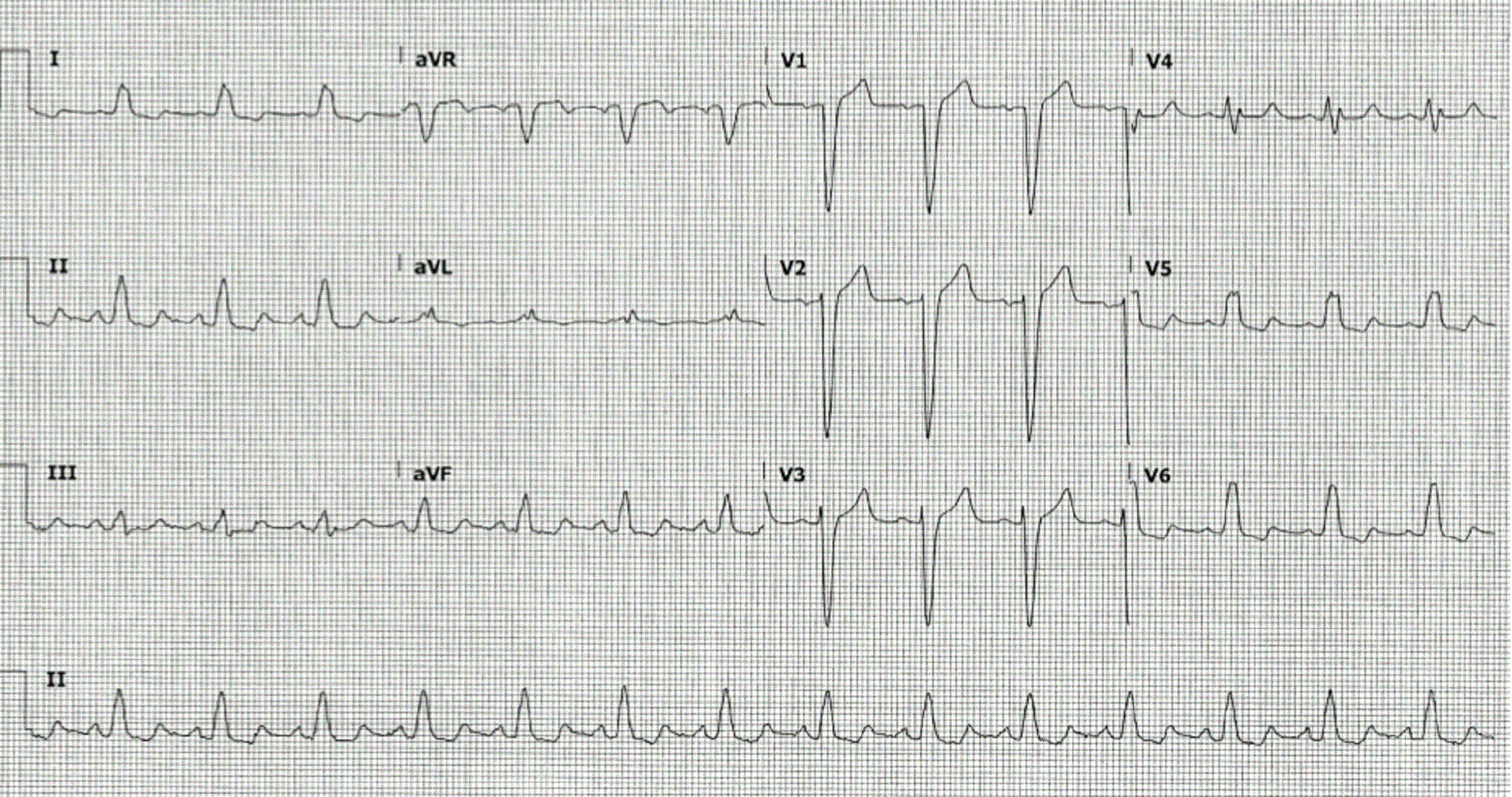
Figure 1. Twelve lead electrocardiogram with sinus rhythm with left bundle branch block.
CMR Findings
A CMR with only cardiac strain analysis-based research investigation was carried out in this patient to further establish a diagnosis of cardiotoxicity. Traditional CMR sequences, including cine SSFP, T1/T2 mapping, and delayed enhancement were not performed. Comprehensive analysis of her LV contractile parameters included MRI-based computations of 3D myocardial strains and torsion to predict the extent of dysfunction in a manner more quantitatively detailed than conventional LVEF measurements. CMR data was obtained with the Displacement Encoding with Stimulated Echoes (DENSE) sequence (Movie 1) and 3D strains and torsion analysis conducted with an automated (patented) research algorithm consisting of multi-threshold image quantization for LV boundary detection, displacement analysis via phase unwrapping and strain analysis using a rapid meshfree methodology [1-5]. This research algorithm, which has been validated over several past studies, was used as a single-scan tool that provided comprehensive and automated analysis of LV chamber quantifications and 3D strain analysis. In this case the analysis required less than six minutes of processing time for 15 short axis slices [1-3]. Immediate signs of cardiac dysfunction can be observed from Figure 2a where a biased and unusual septal twitch becomes obvious during systole compared to a normal systolic contraction in a healthy subject (Figure 2b). The peak systolic longitudinal strain was significantly lower than healthier ranges found in our normal subjects’ database, which were -9% (vs -14 ± 4%), -13% (vs -19 ± 3%), -14 (vs -22 ± 4%) for the basal, mid-ventricular and apical LV regions, respectively. Similarly, peak systolic torsions were significantly different from the ranges found in the healthy subjects, which were 4.1 (vs 8.8 ± 1.7 ) radians for the mid-ventricular and 5.5 (vs 10.6 ± 1.6 ) for the apical LV regions (both with respect to the basal region). The lowered peak systolic torsions in comparison to a healthy subject can be seen in Figures 3 and 4.
Movie 1. Displacement Encoding with Stimulated Echoes (DENSE) sequence mid short axis.
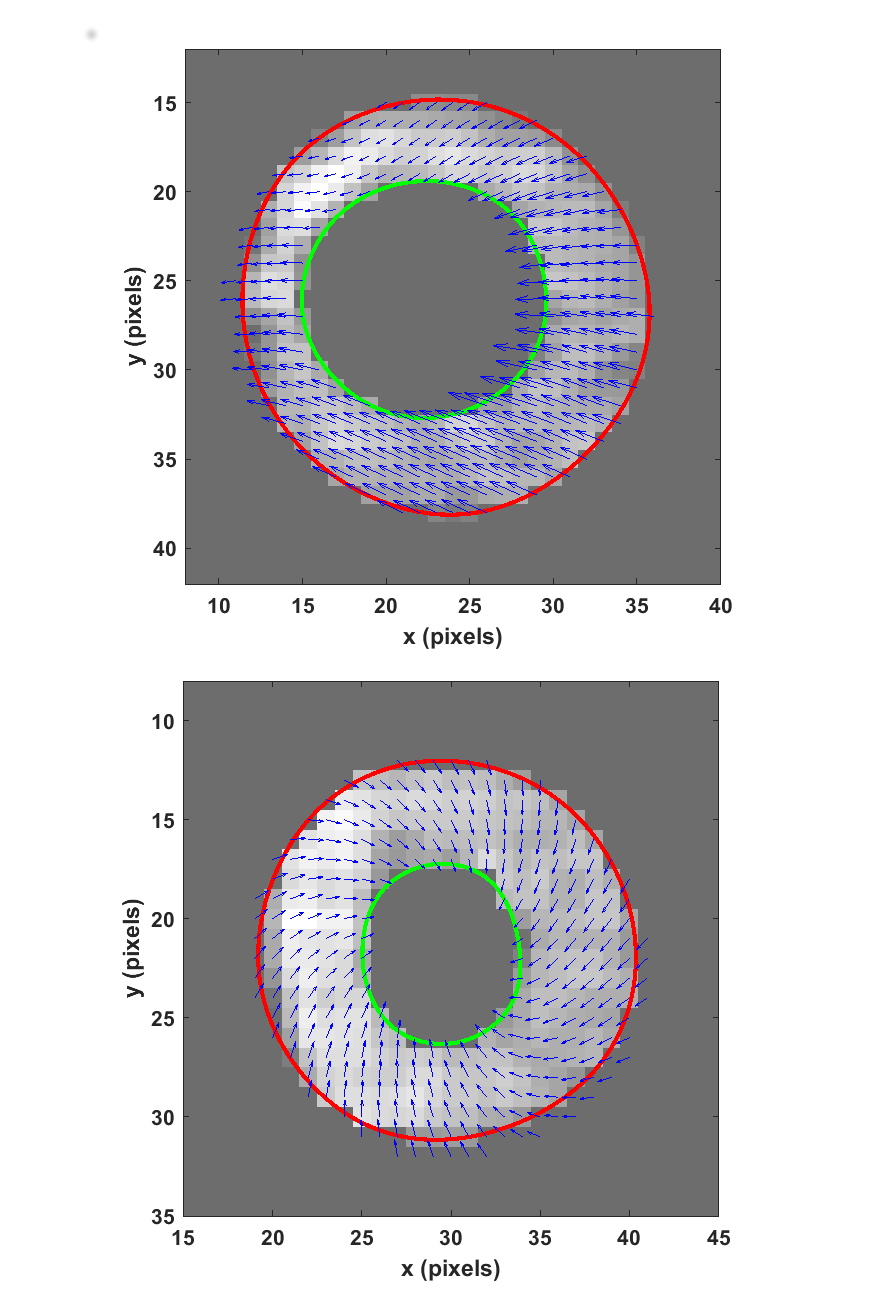
Figure 2. Systolic displacement vectors in (a) the patient’s and (b) a healthy volunteer’s mid-ventricle.
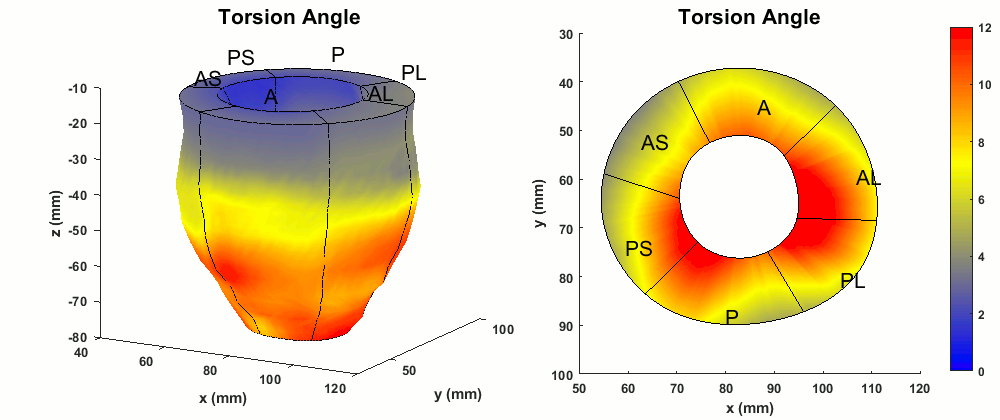
Figure 3. Typical normal ranges of torsion (degrees) in the whole LV and a mid-ventricular short-axis slice of a healthy volunteer.
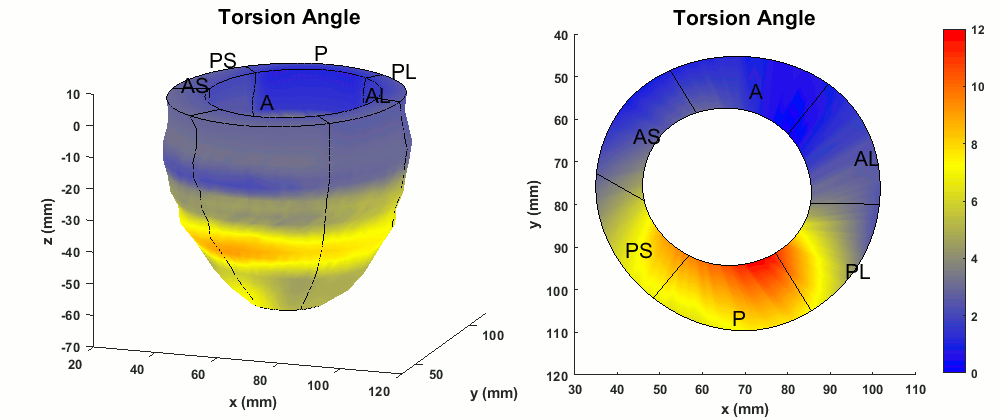
Figure 4. Abnormal torsion (degrees) patterns in the whole LV and a mid-ventricular short-axis slice of the patient.
Conclusion
Cardiotoxicity is a likely diagnosis given the patient’s age, chemotherapy exposure, and existence of cardiac comorbidities. Furthermore, follow up echocardiogram now shows a LVEF < 25%. Abnormal CMR-based contractile function and strain analysis are strong indicators of cardiotoxicity that were not revealed by the earlier LVEF measurements. It also indicates the possibilities of consistent, long-term assessments of myocardial strain parameters supplementing routine cardiac examinations to support treatment options.
Perspective
In regards to whether the findings of abnormal strain are related to the LBBB, a strong argument can be made that this is a combined deteriorating effect of cardiotoxicity and LBBB, where cardiotoxicity intensifies co-existing cardiac dysfunctions as shown in previous studies [6, 7]. The combined effect of two different dysfunctions is evident in this patient from the biased displacements (Figure 2) found in the entire short axis cross section which is not exclusive to her septum. The existence of the cardiotoxicity is further supported by the reductions seen in global longitudinal strain and torsion in the entire LV, which are parameters shown in previous studies as being effective in detecting cardiotoxicity [8, 9]. Importantly, the follow-up echocardiogram showed a LVEF < 25%, a significant drop between subsequent tests. This conforms to guideline directed medical therapy (GDMT) based diagnosis of cardiotoxicity in breast cancer patients treated with anthracyclines and trastuzumab. Other CMR parameters of cardiotoxicity, T1/T2 contrast mapping showing the diffuse myocardial fibrosis (DMF) that occur with cardiotoxicity, could have mirrored the strain-based mechanisms of finding the underlying dysfunction. [10]. However, the uncertainty remains that her LV dysfunction could entirely be an effect of LBBB. The newly patented algorithm for DENSE-based strain analysis used in this case is available via an application with user interface where automated analysis typically takes five minutes in a patient, validated against the gold standard of tissue tagging [1, 2, 5]. Hence, the DENSE strains are expected to be similar to those measured clinically with echocardiogram and HARP, and HARP protocols are commonly validated in comparison to tissue tagging. Echocardiogram strain measurements as recommended by the American Society of Echocardiography (ASE) for post-chemotherapy assessments (including associated dose adjustment) would have added more robustness to this case [11]. DENSE is best suited for imaging systolic function in cardiotoxicity as it is fast with high resolution unlike CMR tissue tagging. DENSE does not suffer from excessive artifacts, noise, or decreased resolution due to the asymmetrically sampled spectral peak also found in HARP. The repeatability and reproducibility of DENSE data is independent of operator accuracy, a common challenge in echocardiographic strain [1]. Ultimately, many breast cancer patients who have been cured via chemotherapy may suffer from progressive LV dysfunction due to chemotherapy induced cardiotoxicity and symptomatic heart failure (HF)[7-10, 12]. These studies repeatedly emphasize that despite recent advancements in HF treatment, a majority of patients with CA-induced cardiac dysfunction show no improvement in LV function, and that the benefits of detecting cardiotoxicity early in its manifestation (not always apparent from small declines in LVEF) cannot be ignored.
A study by Siedman et al. which shows the abnormal septal motion in systole that undermined treatment efficacy when patients were administered a combined dose of CAs including anthracyclines, trastuzumab and cyclophosphamides [12]. In this context, studies also show that myocardial dysfunction measured with global and regional computations of 3D strains (and torsion) are more sensitive toward early detection of cardiotoxicity prior to any LVEF evaluations. Additionally, the ability to detect cardiotoxicity more sensitively via analysis of strain parameters (longitudinal strain and torsion) was shown by two previous studies[8, 9]. Hence, detecting cardiotoxicity related dysfunction via CMR DENSE-based strain analysis is a viable methodology that should be practiced more in mainstream clinical diagnosis of CA induced cardiomyopathy toward further improving the quality of surveillance required in this patient population.
1. Kar J, Knutsen AK, Cupps BP, Pasque MK: A validation of two-dimensional in vivo regional strain computed from displacement encoding with stimulated echoes (DENSE), in reference to tagged magnetic resonance imaging and studies in repeatability. Ann Biomed Eng 2014, 42:541-554.
2. Kar J, Knutsen AK, Cupps BP, Zhong X, Pasque MK: Three-dimensional regional strain computation method with displacement encoding with stimulated echoes (DENSE) in non-ischemic, non-valvular dilated cardiomyopathy patients and healthy subjects validated by tagged MRI. J Magn Reson Imaging 2015, 41:386-396.
3. Kar J, Zhong X, Cohen MV, Cornejo DA, Yates-Judice A, Rel E, Figarola MS: Introduction to a mechanism for automated myocardium boundary detection with displacement encoding with stimulated echoes (DENSE). Br J Radiol 2018:20170841.
5. Kar JC, B.P., Pasque MK. Systems and methods for measuring cardiac strain. United States: Washington University; 2018.
11. Addetia K, DeCara JM. Caring for the cardio-oncology patient: straining to foresee the future. J Am Soc Echocardiogr 2015, 28:515.
Please click here to view the case on CloudCMR.
Case prepared by:
Jason N. Johnson MD MHS
Associate Editor SCMR Case of the Week
Le Bonheur Children’s Hospital
University of Tennessee Health Science Center







