Manuel Gutierrez, Monika Arzanauskaite, Marousa Ntouskou, Ahmed Kharabish
Liverpool Heart and Chest Hospital NHS Foundation Trust, Liverpool, United Kingdom
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical history:
A sixty-eight-year-old woman, a former smoker, presented to the Emergency department after twenty-four hours of malaise, sore throat and chest pain with a pleuritic component. There was no other relevant past medical history. She was afebrile without other symptoms. Physical examination revealed audible crackles at bilateral lung bases with associated hypoxia. Electrocardiogram (ECG) performed as part of the chest pain assessment demonstrated widespread ST depression in the inferolateral leads as well as Q waves and ST elevation in I and aVL. Portable echocardiography showed a severely impaired left ventricular function with global severe hypokinesia of mid segments, hypokinesia of apical segments and a hyperdynamic base. Both cardiac troponins and creatinine kinase were severely elevated (Troponin T 1020 [<14ng/L]; CKMB 14 µg/L [<5µg/L]). The patient underwent an invasive angiography which did not show significant coronary artery disease. During angiography, she suffered sudden clinical deterioration with severe hypoxemia that improved after administration of high dose furosemide. A portable chest radiograph on ward admission showed features consistent with pulmonary edema. Because of the absence of significant coronary disease on angiography the working diagnosis of myocardial infarction with nonobstructive coronary arteries (MINOCA) was established. Urgent cardiac magnetic resonance was requested.

Figure 1 (ECG). ECG on admission showing ST depression in the lateral wall (V4-V6), T wave inversion in V1 and Q waves and ST elevation in I and aVL.

Figure 2 (chest radiograph). Portable chest radiograph after catheterization and admission on the ward showing bilateral, peri-hilar airspace opacities and Kerley B lines consistent with acute pulmonary edema.
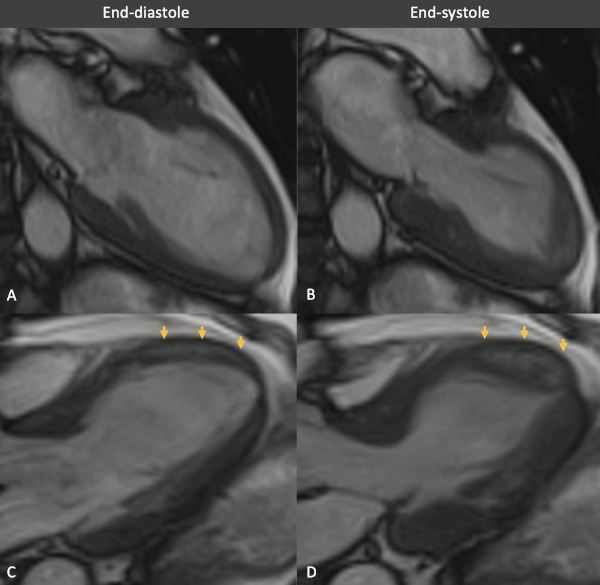
Figure 3 (MRI, cine sequences snapshots). Vertical long axis (VLA, A-B) and left ventricle outflow tract (LVOT, B-C) views of balanced SSFP cine in end-diastolic (left column) and end-systolic (right column) phases show a wall motion abnormality in the mid anterior and anteroseptal walls. Note the mid to apical mid-wall myocardial hyperintensity (arrows).

Figure 4 (T2-STIR & LGE). T2-STIR (upper panel) and late gadolinium enhancement (LGE) (lower panel) sequences in basal (left column -A,D), mid-ventricular (central column – B,E) and apical (right column – C,F) short axis (SA) views. Although relatively subtle, on STIR images there is myocardial hyperintensity (green asterisks) in the basal to apical anterior and lateral walls and mid anteroseptum, which is suggestive of myocardial edema. On LGE images there is subepicardial enhancement (arrows) in the basal to mid antero- and inferoseptum. Of note, the existence of epicardial fat (blue asterisks) might be confounded by subepicardial enhancement. Typically, epicardial fat appears hyperintense on the LGE sequence but is suppressed on STIR images, as seen in the top panel.

Figure 5 (T1 & T2 maps). Horizontal long axis native T1 and T2 maps. On native T1 map (left) two areas of remarkably shortened T1 time are noted in the apical septum and apical lateral wall. As opposed to the previous tissue characterization images, the T1 time shortening is evident here. The T2 map (right) shows no obvious abnormalities.
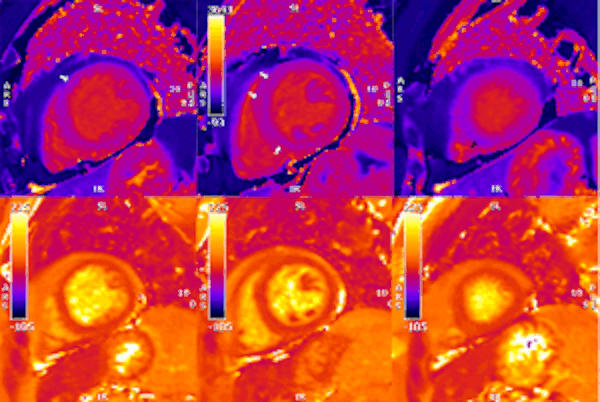
Figure 6. Short axis native T1 and T2 maps. As with LGE sequence, in the native T1 map (upper row) there is an impression of longer times in the subepicardial basal anteroseptum and mid-wall mid-ventricular septum (white arrows). In the apical slice, there is a small area of subtle shortened T1 time in the lower part of the septum (black arrow). T2 times (lower row) appeared unremarkable in all segments.
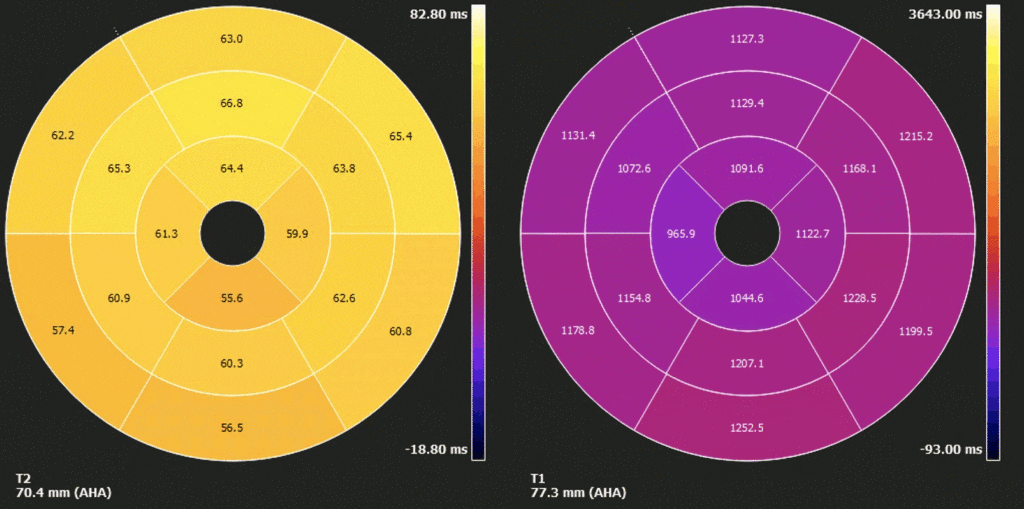
Figure 7. Polar map of native T1 and T2 times in myocardial segments. Diffusely elevated native T1, except where focally decreased in the apical septum. Diffusely borderline high/ mildly elevated T2 times.

Figure 8. Horizontal long axis (HLA) T2-STIR, early gadolinium enhancement (EGE) and late gadolinium enhancement (LGE) sequences. Subtle, faint areas of mid-wall apical hypointensity (yellow arrows) can be seen in all three sequences .

Figure 9. Previous abdominal CTs. The heart had been partially covered in two previous abdominal scans related colonic pathology. (2018 – right, and 2016 – left). On both scans a focal myocardial hypodensity is visible in the same segments with abnormal signal intensity on CMR. The CT density is similar to the one showed by the pericardial and subcutaneous tissue.
CMR Findings:
Myocarditis protocol was performed at 1.5T (Siemens Aera). Cine images (Figure 3) revealed severe mid-ventricular hypokinesia in keeping with the findings of the portable ultrasound on admission. The left ventricular ejection fraction at the time of the CMR (24h after admission) was moderately reduced (LVEF 44%).
On T2 sequences (Figure 4), a mildly increased signal intensity was present predominantly in the mid anteroseptal and mid to apical anterior and lateral walls (Figure 4). Although there were no visually apparent areas of lengthened T2 times on T2 maps (Figures 5 and 6), a derivate segmental polar map showed diffusely borderline high/ mildly elevated T2 times (55.6-66.8ms – Figure 7). On LGE images, there was subepicardial and mid-wall enhancement in the basal to mid septal segments (Figure 4) in a non-infarct pattern. These features were most consistent with active myocardial inflammation due to myocarditis although concomittant fibrosis cannot be excluded. The clinical presentation is less consistent with stress cardiomyopathy, but this entity may also produce abnormal T1 and T2 signal on CMR due to edema and inflammation.
Surprisingly, on native T1 maps (Figures 5 and 6) there were apical areas of shortened T1 time (mean 800ms +/- 44.6ms). Careful review of other tissue characterization sequences (T2 STIR, EGE, LGE) allowed the identification of subtle areas of low mid-wall signal intensity matching the territories with shortened T1 times (figure 8).
As documented in patients with acute myocardial infarction, the existence of short T1 times and a hypointense core within areas of edema or LGE are suggestive of microvascular obstruction or myocardial haemorrhage (1). Nonetheless, in our patient there were some inconsistencies. Often, the hypointense core is easily visible and, more importantly, T2 times should be clearly abnormal. In contrast, the features in the apex (hyperintensity on bSSFP, subtle hypointense core after fat saturation and contrast administration, low T1 but normal T2 times) fit with the presence of intramyocardial fat in the context of myocardial inflammation most likely due to acute myocarditis, although stress cardiomyopathy may be possible (mild diffuse myocardial edema, native T1 increase diffusely, and areas of delayed enhancement).
Conclusion:
Given the clinical picture of atypical chest pain, LV dysfunction, ST segment changes on ECG, documented myocardial damage and consistent MR findings (abnormal native T1, non-ischemic LGE and edema) the diagnosis of myocarditis was made. No specific etiology was identified. Stress cardiomyopathy is possible but less likely clinically. The abnormal appearances on bSSFP images and abnormal T1 times were deemed as incidental presence of myocardial fat. This was confirmed after comparing to the previous abdominal CT studies where the heart had been partially covered; on these studies there were areas of focal low attenuation in the same apical segments described in the CMR study (Figure 9).
Perspective:
The diagnostic workup of acute myocardial infarction without obstructed coronary arteries is not always straightforward as it encompasses multiple etiologies from overlooked coronary artery disease to non-coronary acute myocardial damage (i.e. myocarditis)(2). CMR is the mainstay in the diagnosis of suspected myocarditis. Tissue sampling using endomyocardial biopsy is not often performed due to the invasive nature of the procedure (3), so it is common practice to accept the diagnosis of myocarditis when the Lake Louise criteria (revised in 2018) (4) are met. These include: T1 criteria (non-ischemic pattern of LGE enhancement, lengthened T1 time or increased extracellular volume) and T2 criteria (myocardial edema on STIR, lengthened T2 times). Importantly, both T1 and T2 criteria have to be present. These were identified in the case of our patient who presented with clinical, biochemical, electrocardiographic and CMR compatible findings.
However, the existence of intramyocardial fat played a confounding role initially raising the possibility of inflammation associated with severe damage. Lipomatous metaplasia of the myocardium following myocardial infarction, fibro-fatty replacement of the myocardium in arrhythmogenic ventricular cardiomyopathy, fat-containing intramyocardial lesions in tuberous sclerosis and intramyocardial lipomas are known entities of macroscopic fat tissue within the myocardium. It is also important to note that the presence of non-pathological ectopic myocardial fat is relatively common in healthy adults, and has no clinical implications (5). Based on one cardiac CT study an incidence of up to 11% has been estimated (6). The most usual location is the right ventricle (free wall, RVOT, moderator band or apex) however, it can also be found in the interventricular septum or left ventricular apex. It is important to recognize this incidental fat as the features can sometimes be misleading (e.g. forming a small mass in the case of adipose degeneration of the moderator band or myocardial damage as in our case) and to correlate its presence to patient’s presentation and clinical history. On CMR, fat can sometimes have similarities with MVO/ hemorrhage, as both fat and MVO/hemorrhage cause T1 time shortening. On T2 STIR, saturated fat can give the impression of a hypointense core. Nevertheless, this appearance will be subtle whereas when the microcirculation is disrupted or there are haemoglobin products the hypointense core will generally be prominent and surrounded by obviously abnormal myocardium. The confirmation can be obtained on T2 and T2* maps: the times will be normal in cases of intramyocardial fat but abnormally low in MVO/haemorrhage. Whenever possible, previous imaging such as body CT studies will help to confirm the presence of fat, as fat has a characteristically low attenuation of −20 to −130 Hounsfield Units on CT, similar to the epicardial or the subcutaneous adipose tissue.
Link to full case image series:
https://www.cloudcmr.com/0757-
Bibliography
Case prepared by:
SCMR Case of the Week Editorial team





