Anna Baritussio, MD, PhD1,2, Estefania De Garate Iparraguirre, MD2 , Chiara Bucciarelli-Ducci, MD, PhD2
1 Department of Cardiac, Thoracic, Vascular Sciences, and Public Health, University of Padua, Padua, Italy
2 Bristol Heart Institute, NIHR Bristol Biomedical Research Centre University Hospitals Bristol NHS Foundation Trust and University of Bristol, Bristol, United Kingdom
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical history
A 34 year old naval officer, otherwise fit and healthy, with no known family history of cardiomyopathies, presented with chest pain (CP), poorly tolerated atrial fibrillation and progressive hypotension. An echocardiogram showed asymmetric septal hypertrophy, with preserved left ventricular ejection fraction (LVEF) and severe left atrial enlargement. As high sensitive Troponin I (hs-TnI) was elevated, with persistent CP, a coronary angiogram was performed showing unobstructed coronaries and a chest CT ruled out pulmonary embolism. Blood tests showed mildly increased inflammatory markers. A cardiovascular magnetic resonance (CMR) was requested.
CMR findings
The left ventricle (LV) had normal volumes (indexed end-diastolic volume 86 ml/mq, indexed end-systolic volume 38 ml/mq) with mildly reduced LVEF (56%). There was a mixed pattern of LV hypertrophy (LVH) and thinning, with asymmetric hypertrophy in portions of the anterior and septal segments (Fig.1, white asterisk), from base to mid-cavity (max 15mm basal), and wall thinning of the basal and mid-cavity lateral wall, extending to the apical segments (min 4mm apical anterior wall); hypertrophied segments appeared stiff, while the thinned segments were mildly hypokinetic. Two large myocardial crypts were noted in the basal anterior and anterolateral walls (Fig.1, white arrows).
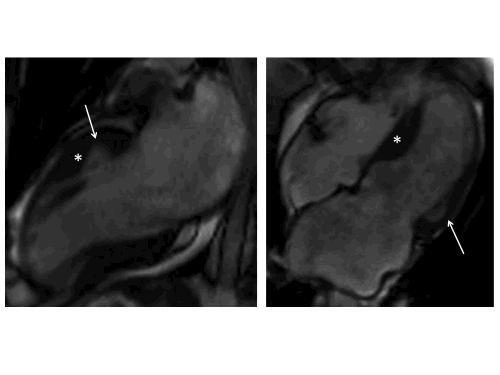
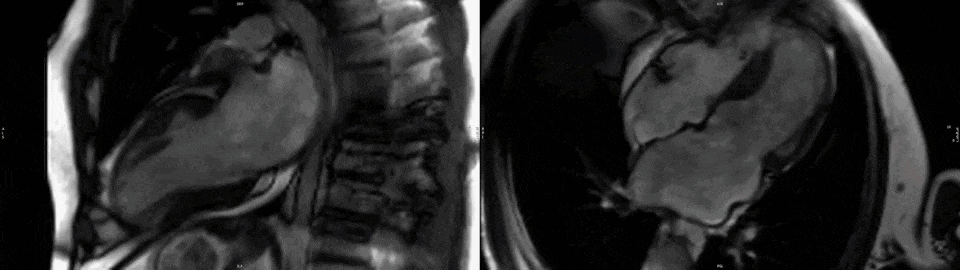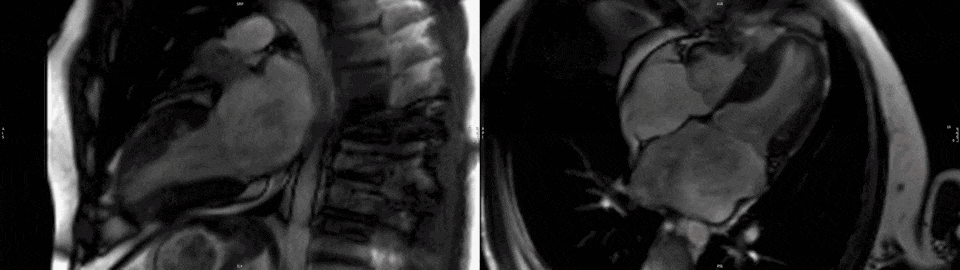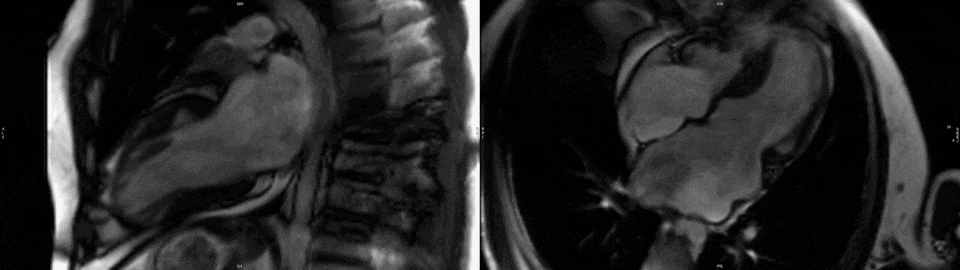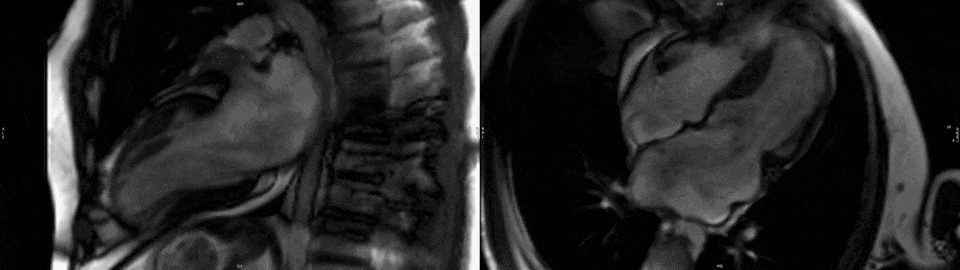
Figure 1. Top row. Two (left panel) and four chamber (right panel) SSFP cine sequences showing septal and anterior LVH (white asterisk) and myocardial crypts of the basal anterior and anterolateral walls (white arrow). Bottom row. Corresponding two (left panel) and four chamber (right panel) SSFP moving cine images.
The right ventricle had normal wall thickness, volumes (indexed end-diastolic volume 74 ml/mq, indexed end-systolic volume 24 ml/mq) and systolic function (RVEF 65%). The left atrium was severely dilated (50cm2). On T2-STIR imaging, there was mid-wall myocardial edema of the basal inferoseptum (Fig.2, white arrows), which corresponded to an area of higher T2 values on T2 mapping (Fig.2 black arrow).
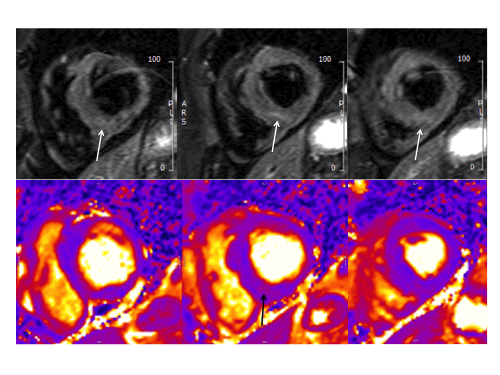
Figure 2. Upper panel: Basal short axis T2-weighted sequences showing mid-wall increased signal intensity in the basal inferoseptum, consistent with myocardial edema (white arrows). Lower panel: Basal short axis T2-mapping images showing increased T2 values in the basal inferoseptum (black arrow).
On post-contrast images, patchy mid-wall myocardial LGE was noted in the inferoseptum (Fig.3, white arrows), from base to apex, and in the mid-cavity and apical anterior wall (Fig.3, black arrows). On native T1 mapping, increased T1 values were noted, especially in the hypertrophied inferoseptum (Fig 3, right panel, black arrow). Mediastinal lymphadenopathy was also noted.
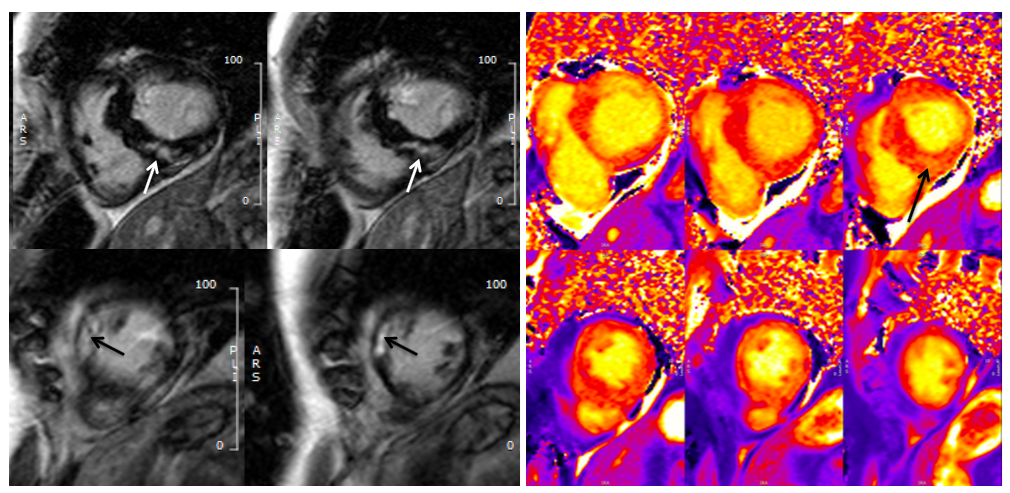
Figure 3. Left panel: Basal to mid-wall short axis post-contrast sequences showing patchy mid-wall myocardial LGE of the inferoseptum (white arrows), and in the mid-cavity anterior wall (black arrows). Right panel: Short axis native T1 mapping images, showing increased native T1 values in the basal inferoseptum (black arrow).
Conclusion
CMR findings were consistent with an underlying cardiomyopathic process. The presence of asymmetric septal and anterior hypertrophy with non-ischemic fibrosis, could lead to a diagnosis of HCM with replacement fibrosis, but cardiac sarcoid, especially with evidence of septal edema and mediastinal lymphadenopathy, could also be possible. An infective process could also be possible, given the presence of reactive edema in the hypertrophied segments, mediastinal lymphadenopathy and increased inflammatory markers, although it is usually less likely related to LVH.
However, there was an unusual pattern of LVH and wall thinning, which is frequently described in mitochondrial cardiomyopathies. Barth’s syndrome for example, which is secondary to an X-linked related mitochondrial dysfunction, is sometimes characterized by transition between dilated and hypertrophic cardiac morphology (1).
A suspicion of mitochondrial cardiomyopathy was raised, and given persistence of CP with elevated hs-TnI, an endomyocardial biopsy (EMB) was performed, showing hypertrophied myocytes with focal interstitial fibrosis (Fig.4, black asterisk), not consistent with hypertrophic cardiomyopathy (HCM), sarcoidosis or amyloidosis, and with an increased number of mitochondria within the myocytes on electron microscopy, the latter being frequently reported in mitochondrial cardiomyopathies.
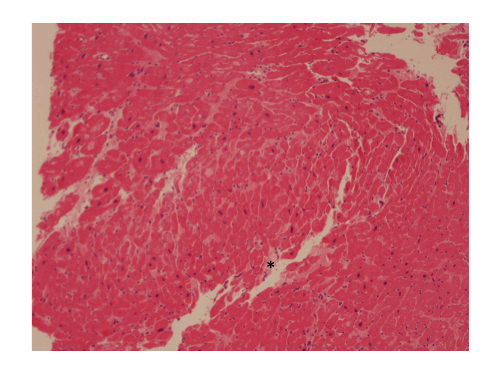
Figure 4. Endomyocardial biopsy showing hypertrophic myocytes with interstitial fibrosis (black asterisk).
Perspective
Myocardial LVH is the final expression of different cardiomyopathies, with widely different prognosis and requiring different management strategies. CMR has a pivotal role in differential diagnosis of hypertrophic phenotypes due to its unique tissue characterization properties (2); it is a powerful diagnostic tool, which should however never be separated from patients’ clinical history, laboratory tests, physical examination and other imaging findings.
The unusual pattern of LVH and wall thinning raised the suspicion of mitochondrial cardiomyopathy, leading to EMB that excluded HCM and sarcoid, while showing an increased number of mitochondria, which is frequently described in mitochondrial cardiomyopathies. Although rare, mitochondrial cardiomyopathies are characterized by a wide range of cardiac abnormalities, ranging from conduction abnormalities, to hypertrophic or dilated phenotypes, with variable LGE patterns, such as mid-wall basal inferolateral wall or diffusely distributed LGE.CMR aids in the diagnosis of mitochondrial cardiomyopathies, with relevant implications on patients’ management given the increased mortality risk associated with this disease (3).
Based on EMB and CMR findings, a diagnosis of mitochondrial cardiomyopathy seems likely and should prompt wider genetic testing as further diagnostic work-up.
Click here to review this case on Cloud CMR.
References
Case prepared by:
Anna Baritussio, MD PhD
Department of Cardiac, Thoracic, Vascular Sciences, and Public Health, University of Padua, Padua, Italy
Bristol Heart Institute, NIHR Bristol Biomedical Research Centre University Hospitals Bristol NHS Foundation Trust and University of Bristol, Bristol, United Kingdom
SCMR COTW Editorial Team







