Kana Fujikura, MD PhD1, Charles W. Benton, RT(R)(CT)(MR)1
National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, Bethesda MD, USA1
Calin V. Maniu, MD
Cardiology, Johns Hopkins Community Physicians – Heart Care, Bethesda MD, USA
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical History
70 year-old female nursing home resident with past medical history of hypertension, hypothyroidism, peripheral vascular disease, and obsessive-compulsive disorder presented to emergency department with shortness of breath of supposedly several days duration. She denied chest pain, palpitation, or cough.
Upon arrival to the emergency department, she was in respiratory distress. Her blood pressure was 102/66 mmHg, heart rate was 108 bpm, SpO2 88% on room air. She was afebrile and frail – weight 40 kg (BMI 17 kg/m2). She was tachypneic with accessory respiratory muscle use. Rhythm was regular, 2/6 systolic murmur was noted at the base. She had +2 bilateral ankle edema. Chest X-ray showed bilateral diffuse infiltrates. She was placed on bi-level positive airway pressure (BiPAP) with 100% FiO2, and her respiratory status improved with SpO2 of 96%.
Laboratory examination showed mild anemia with hemoglobin 10 g/dL (normal > 12 g/dL), creatinine 1.4 mg/dL (normal < 1.2 mg/dL) and BUN 47 mg/dL (normal < 30 mg/dL) [3 years prior creatinine was 0.9 mg/dL and BUN 29 mg/dL) and eGFR 38 mL/min/1.73m2 [3-years prior eGFR >60 mL/min/1.73m2], elevated troponin T 2.77 ng/mL (normal < 0.01 ng/mL), and severely elevated pro-brain natriuretic peptide (pro-BNP) >70,000pg/mL (normal < 125 pg/mL). Her COVID-19 PCR test was negative. Electrocardiogram showed sinus tachycardia with old inferior myocardial infarction and non-specific T-wave changes in inferior and lateral leads (Figure 1). The diagnosis of non-ST elevation myocardial infarction was entertained along with heart failure.
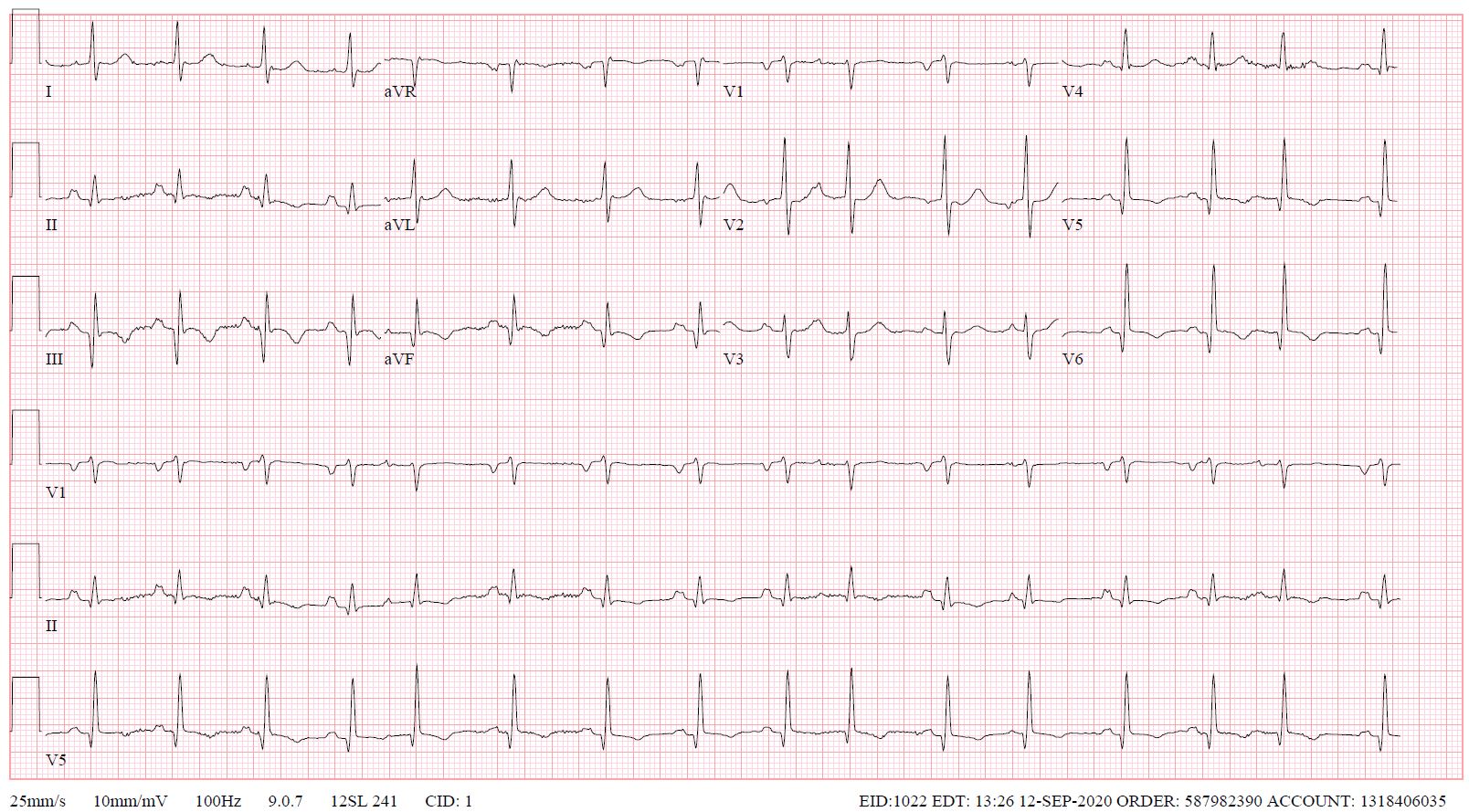
Figure 1. ECG on admission.
On echocardiogram, the left ventricular (LV) size was normal and systolic function was moderately decreased, ejection fraction (EF) 35% with significant regional wall motion abnormalities consistent with inferior MI. The mitral valve leaflets were bilaterally tethered, resulting in severe mitral valve regurgitation (Figure 2). The aortic valve was calcified with stenosis and regurgitation. The mean pressure gradient across the aortic valve was 21 mmHg, with maximum aortic valve velocity 3.3 m/s and calculated aortic valve area of 0.9 cm2 (aortic valve area index 0.7 cm2/m2) suggestive of at least moderate aortic stenosis (Figure 3). She had pulmonary hypertension with estimated right ventricular systolic pressure of 60 mm Hg. An invasive cardiac evaluation was recommended but the patient opted for a conservative strategy with medical management. Upon her request, palliative care team was consulted and she elected to have a DNR/DNI status. She was medically managed and discharged home after 14 days of hospitalization with aspirin, clopidogrel, metoprolol succinate, atorvastatin, and furosemide. Angiotensin converting enzyme inhibitor was held for hypotension.

Figure 2. Color Doppler echocardiography of (A) apical 2 chamber and (B) apical 3 chamber views showing severe mitral regurgitation and turbulence flow at the aortic valve.
As her shortness of breath worsened she returned to the emergency room 9 days after her initial discharge requesting full treatment options including invasive procedures. Invasive coronary angiography showed severe multi-vessel coronary disease with chronic occlusion of the dominant right coronary artery (RCA) filling retrograde through contralateral collaterals, subtotal thrombotic occlusion of a small caliber left circumflex (LCx) artery with TIMI 1-2 flow, and probable significant disease in the proximal left anterior descending artery and proximal large diagonal ( Figure 3). LVEDP was 22 mm Hg. By pullback method, mean gradient across aortic valve was 26 mmHg with a peak–to–peak gradient of 22 mmHg.
Ischemic cardiomyopathy was considered as the major contributor for her severe mitral regurgitation and recurrent heart failure. Cardiothoracic surgery team was consulted for possible coronary artery bypass grafting (CABG), aortic valve replacement and mitral valve repair/replacement.The surgical team was of the opinion that she was a high surgical risk, being especially concerned about her frailty. Cardiac magnetic resonance imaging was ordered to assess myocardial viability as part of her risk stratification (and to plan for the surgery).
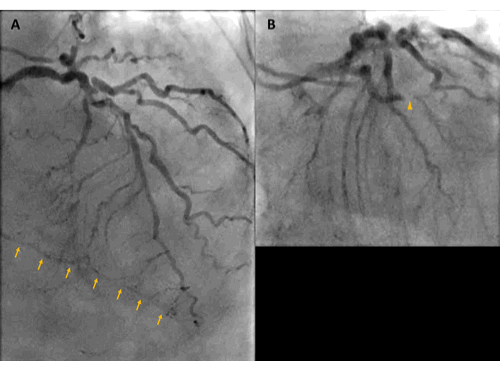
Figure 3. Invasive coronary angiography showing severe multi-vessel coronary disease. (A) AP Cranial view. There was retrograde filling of the right PDA through contralateral collaterals (yellow arrow) indicating chronic occlusion of RCA. (B) LAO-Caudal. LCx is a small caliber vessel with 99% thrombotic subtotal occlusion in its mid segment (yellow arrowhead).
CMR Findings
CMR was performed at 1.5T (Aera, Siemens Healthineers AG, Erlangen, Germany). Cine imaging showed that the left ventricle was severely dilated (LVEDVi 130 mL/m2) and systolic function was severely decreased (LVEF 34 %) (Video 1). In the territories of RCA and LCx, there was a large area of thinned left ventricular wall associated with severe hypokinesis and akinesis, and myocardial infarction of >75% transmural extension (Figure 4). The apical cap was thin and aneurysmal, with signs of a transmural myocardial infarction. There was moderate mitral regurgitation (regurgitation volume was 18 ml and regurgitation fraction was 34 %). Peak aortic valve velocity was 3.1 m/s which was compatible with moderate aortic stenosis. Aortic regurgitation was mild.

Figure 4. Late gadolinium enhancement imaging. There was a large area of myocardial infarction with >75% transmural extension in the territory of RCA and LCx.
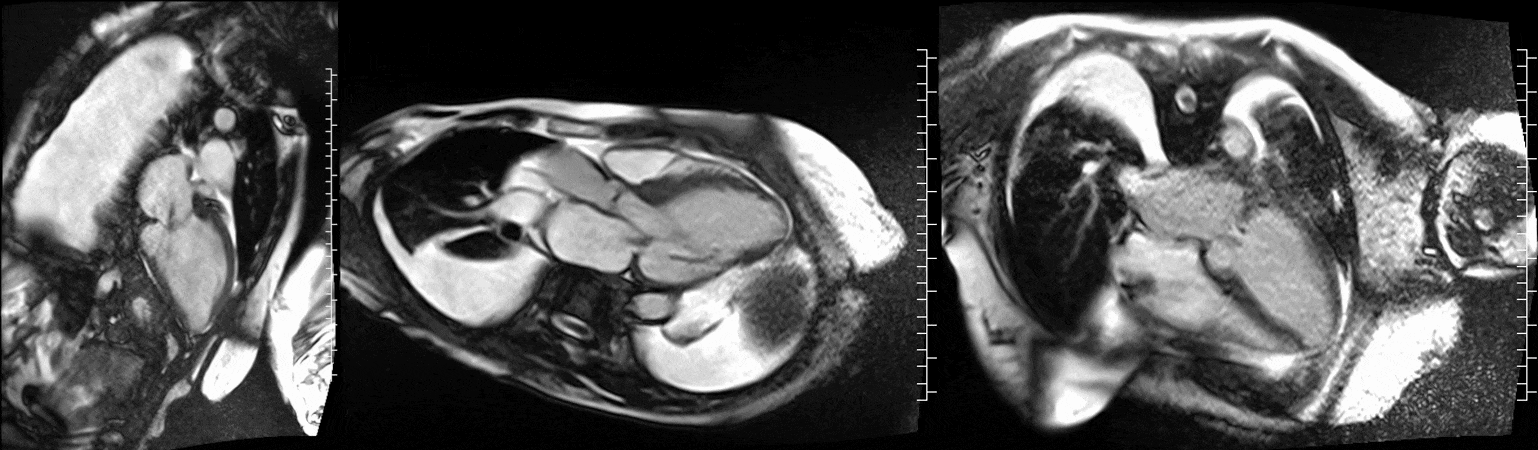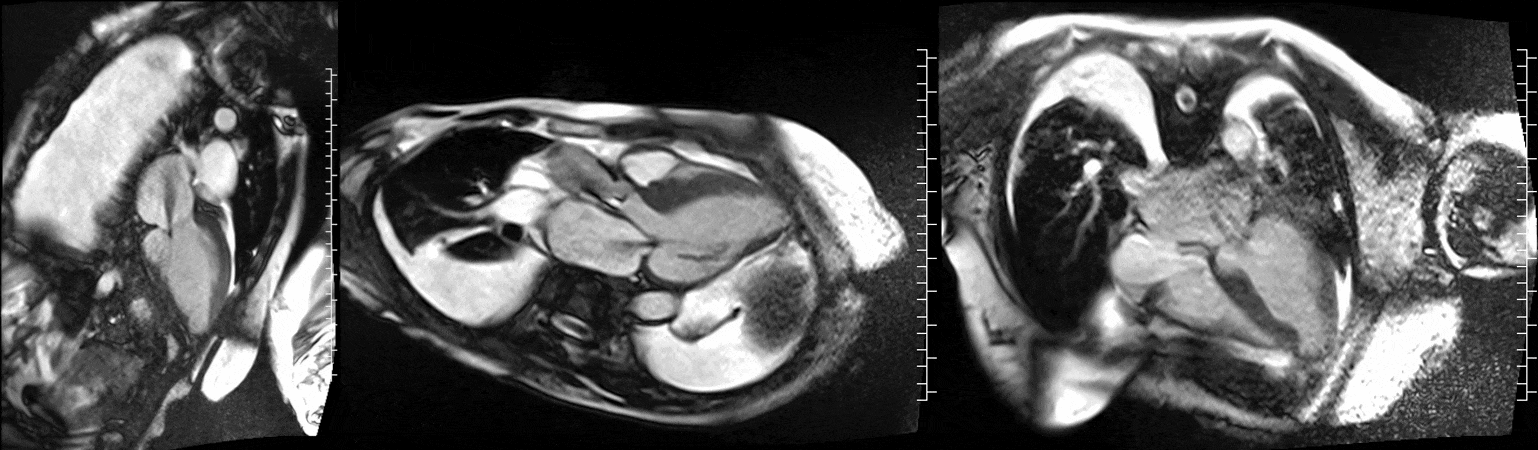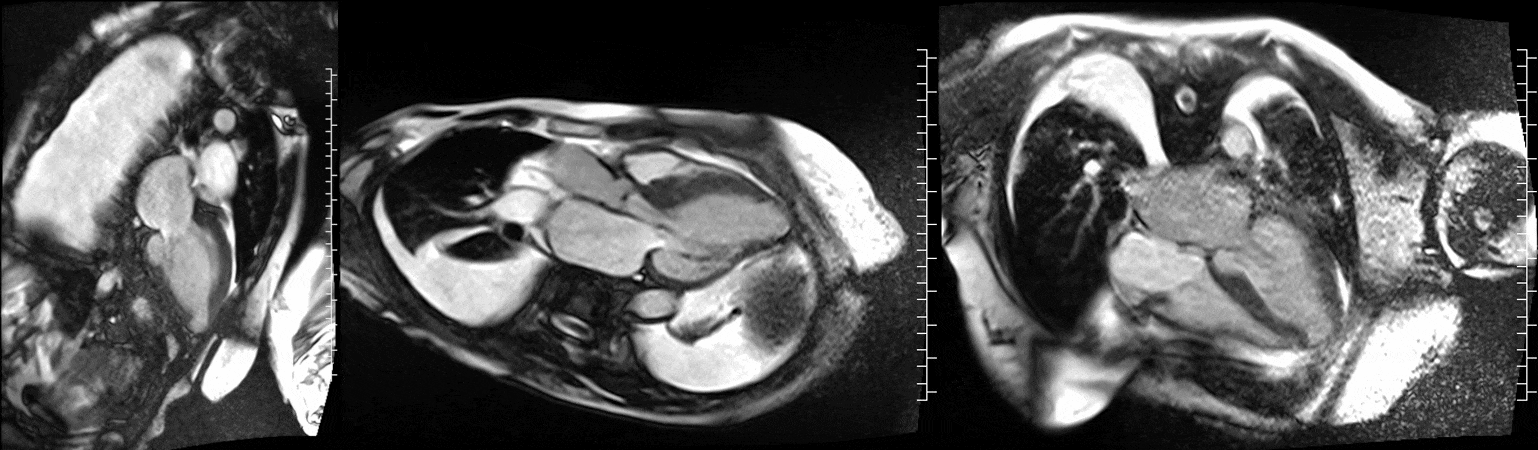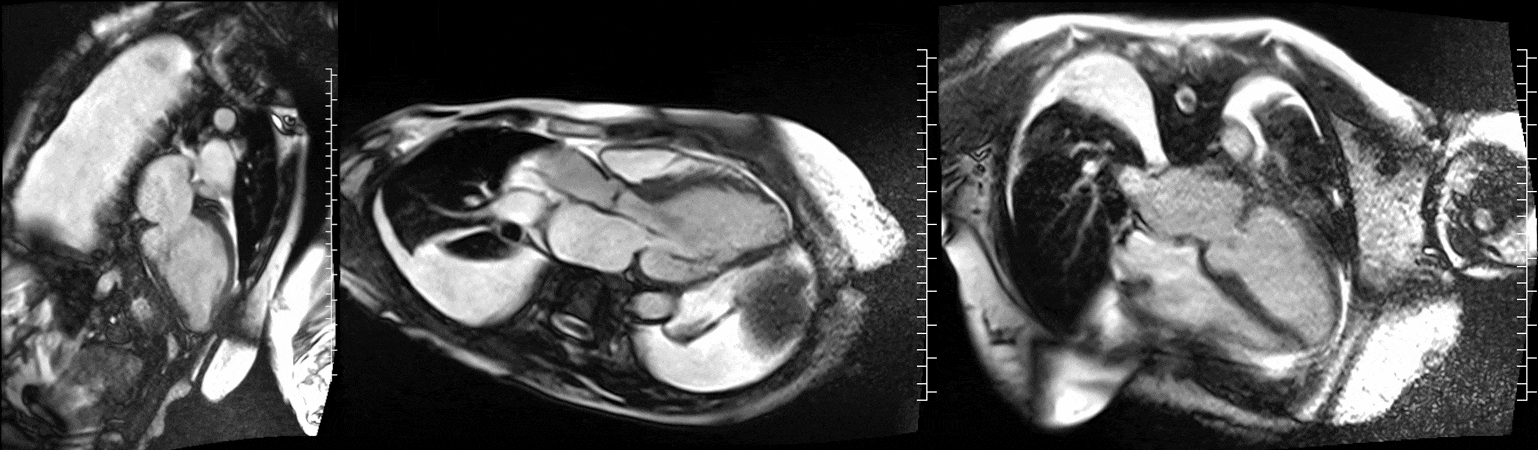
Video 1. Steady-state free precession cine imaging of 4-chamber (left), 2-chamber (mid) and 3-chamber (right panel) views.
Conclusions
A large area in the RCA and LCx territories was thinned with significant transmural myocardial infarction, and thus was considered not viable. Aortic stenosis was at least moderate and mitral regurgitation was moderate.
Based on the lack of viability of a large myocardial territory, the risk/benefit for a surgical intervention with CABG along with valve(s) replacement was deemed unfavorable. The structural heart disease team considered her not to be a good candidate for trans-catheter mitral valve repair (TMVr). The option of trans-catheter aortic valve replacement (TAVR) has been offered but patient decided not to pursue it. The option of percutaneous coronary intervention (PCI) for the LCx and LAD disease was considered. However, revascularization of the presumed non-viable territory perfused by the small LCx was deemed to be of questionable value. PCI of the LAD was considered very challenging due to anticipated difficulty of wiring the diagonal branch and the associated concern of occluding it with stenting only of the LAD. In addition, the clinical impression was that her presentation was related to a significant degree to her valve disease(s) and addressing just the myocardial ischemia had a questionable risk/benefit ratio. H er medical therapy was optimized by adding low dose losartan and ivabradine – as the low dose metoprolol had to be held due to hypotension. At 2-week outpatient follow up she had adequate symptomatic control, albeit at a low, but acceptable for the patient, level of physical activity.
Perspective
This case emphasizes the critical role of CMR in decision making for patient’s clinical management. We presented a case with heart failure related to NSTEMI in the setting of coexisting aortic stenosis and mitral regurgitation. Based on the results of echocardiography and cardiac catheterization, high-risk CABG and aortic valve replacement were considered. However, CMR clarified the complex clinical picture leading to a unanimous agreement among the members of the heart team regarding the viable options for patient’s management, and possibility of Mitral Clip was entertained instead of CABG and aortic valve replacement. The absence of myocardial viability in 2 of the 3 coronary territories for which revascularization was planned, in addition to the other concerning clinical features (especially her frailty), resulted in the surgical team considering the patient too high risk for CABG and AVR.
Transmural extent of late gadolinium enhancement is a well-established method to predict reversibility of myocardial dysfunction [1]. Especially in patients with high risk for surgery, CMR has been frequently utilized to select patients who are most likely to benefit from CABG. In patients with ischemic cardiomyopathy with LVEF ≤ 35%, combination of CABG and medical therapy has been shown to improve long-term mortality compared with medical therapy alone [2]. The applicability of the results of the STITCH trial in clinical practice is not always straightforward as coronary revascularization appears to benefit patients with and without myocardial viability. However, patients without significant myocardial viability are at higher risk irrespective of the treatment strategy. In PARR2 study [3], there were lower cardiac events in patients with LV dysfunction using PET-assisted management based on the amount of viable myocardium compared to standard care, however the results did not reach statistical significance. The lack in significance was considered to be due to low adherence to the PET-assisted recommendations.
CMR has been considered as the gold standard in assessment of ventricular and atrial volume and function [4,5]. There are increasing number of studies evaluating the reliability and accuracy of CMR in valvular heart disease. In aortic stenosis, although echocardiography remains the gold standard, CMR phase contrast imaging provides reproducible results that are concordant with echocardiography [6]. Using steady state free precession and phase contrast imaging, CMR quantification of mitral regurgitation has been demonstrated to be reliably as the difference between left ventricular stroke volume and forward stroke volume [7]. It has been reported that CMR measures mitral regurgitation less severe than echocardiography does [7,8]. Furthermore, post-surgical LV remodeling correlates well with CMR-assessed mitral regurgitation severity but not with echocardiography assessment which is indicative of more accurate assessment by CMR [7].
Management of this case was complicated by coexistence of both aortic stenosis and mitral regurgitation in addition to ischemic cardiomyopathy. Per the guideline for the management of patients with valvular heart disease [8], aortic valve replacement is a class 2b recommendation for patients with moderate AS who are undergoing cardiac surgery. In the absence of surgical coronary revascularization, secondary mitral regurgitation of moderate severity is generally not a surgical indication per se. However, the management of co-existence of the three disease processes (e.g. CAD, AS, and MR) is not well-defined, and is managed on a case-by-case basis.
Click here to view the case on CloudCMR
https://www.cloudcmr.com/1757-1973-0388-0157/
References
Case prepared by
Anna Baritussio, MD, PhD
Department of Cardiac, Thoracic, Vascular Sciences and Public Health
Azienda Ospedale Università, Padua, Italy







