Wejdan Ba-Atiyah MD, Mohammed Omar Galal MD/PhD, Riad Abou Zahr MD*
Pediatric Cardiology section, Department of Pediatrics, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
Clinical History: This is a case of a 4-month-old girl, product of full term pregnancy and birth weight of 2 kg who was postnatally admitted to the neonatal intensive care unit for 25 days at a remote community hospital. She was subsequently discharged home in stable condition. At 3 months of age, she was referred to our center as a case of congenital heart disease.
Upon initial outpatient encounter, the patient’s weight was 2.5 kg with oxygen saturation of 90% in room air. On physical examination, she had subtle dysmorphic features with triangular face, hypertelorism, truncal hypotonia, weak cry and depressed deep tendon reflexes. She had mild tachypnea, a soft systolic ejection murmur at the left upper sternal border and the liver was felt 1 cm below the right costal margin. Her systemic examination was otherwise unremarkable. Electrocardiogram revealed sinus rhythm and left axis deviation. Chest x-ray showed enlarged cardiac silhouette with mild central vascular congestion (Image 1).
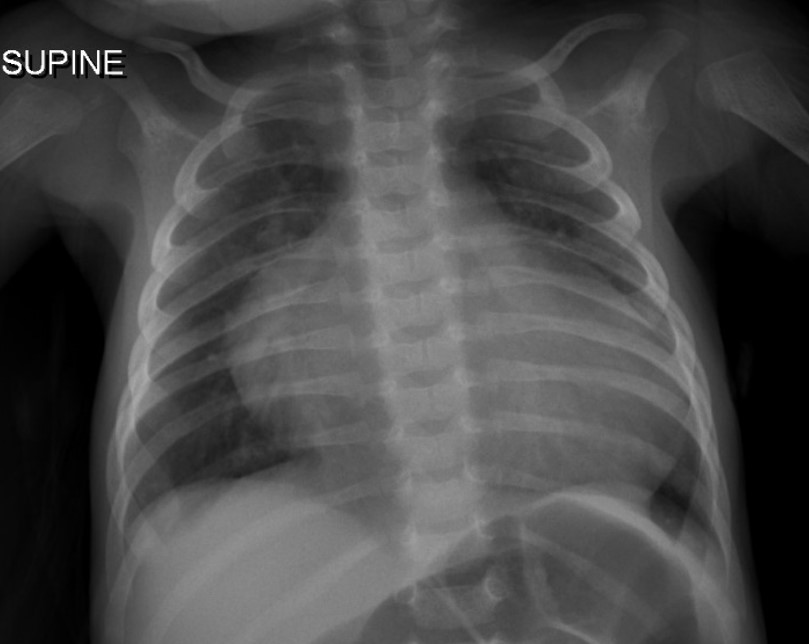
Image 1: Chest x-ray on admission.
Echocardiogram demonstrated situs solitus, levocardia, d-looped ventricles, double outlet right ventricle (DORV) with normally related great arteries, large primum ASD, small secundum ASD, and a moderate VSD. Optimal interrogation of the VSD was technically challenging. The LV was suspected to be hypoplastic with mitral atresia and concern for a huge left atrial appendage aneurysm with to-fro flow associated with that structure (Images 2 and 3, Video 1).

Image 2: Panel A: Left parasternal view demonstrating anteriorly the right ventricle (RV), the presumed hypoplastic left ventricle (LV), and the large posterior aneurysm. Panel B: Subcostal sagittal short axis view of the RV, LV and aneurysm. Panel C: Apical 4 chamber view demonstrating flow into the presumed left atrial appendage aneurysm. Panel D: Apical 4 chamber view demonstrating what was presumed hypoplastic LV and mitral atresia (MA)
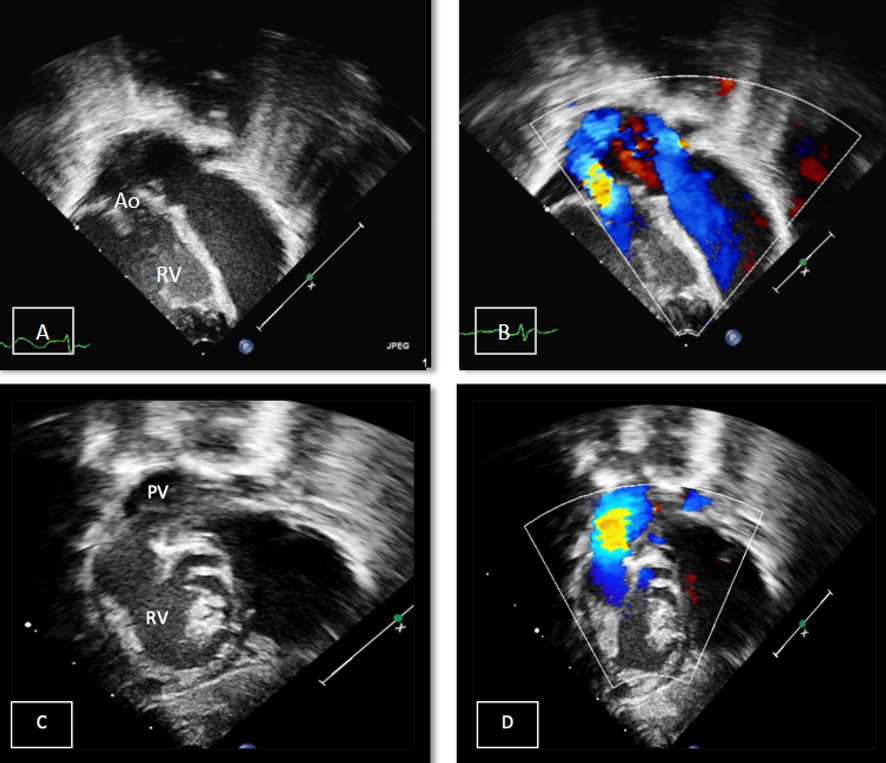
Image 3: Panels A & B: Apical 5 chamber view in 2D and color demonstrating the aorta (Ao) arising from the RV . Panels C & D: Subcostal oblique view demonstrating the pulmonary valve (PV) and artery arising from the RV.
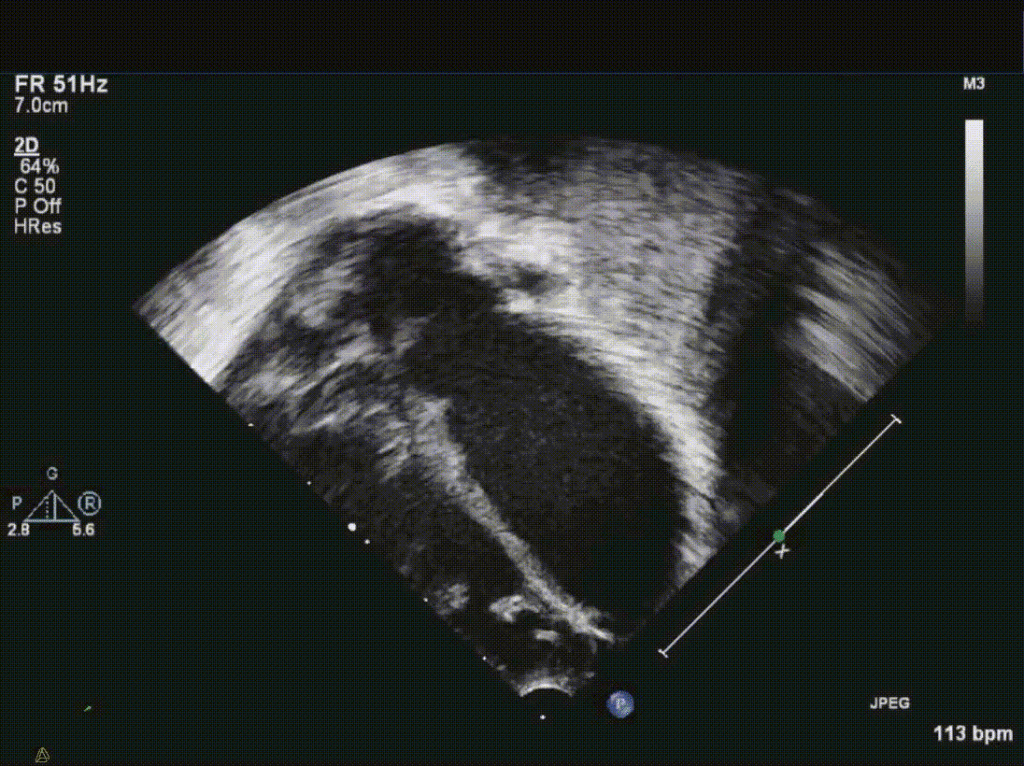
Video 1: Apical echo image demonstrating a large, thin walled, hypocontractile left sided chamber, initially concerning for a possible giant left atrial appendage aneurysm.
Understanding the patient’s diagnosis and hemodynamics was challenging given the atypical left heart findings. For better evaluation of the “aneurysm” a cardiac MRI (CMR) was performed under general anesthesia.
CMR Findings: The study was performed on a 1.5 Tesla magnet with the patient’s heart rate ranging from 91-100 bpm during the study. Cine imaging demonstrated viscero-atrial situs solitus, d-looped ventricles, DORV with normally related great arteries, small (4mm) outlet VSD and left superior vena cava to an unroofed coronary sinus. Both atria were enlarged with a large primum ASD. The left atrioventricular junction was patent with dephasing artifacts across but absent mitral valve apparatus including leaflets, chordae and papillary muscles (Video 2). The left atrial appendage appeared normal.
Video 2: 4 chamber SSFP cine demonstrating a widely patent left atrioventricular junction with dephasing flow artifacts across but absent mitral valve leaflets, chordae and papillary muscles.
Phase contrast cine imaging demonstrated to-and-fro flow across the mitral annulus with E/A waveform reversal suggestive of diastolic dysfunction (Image 4). There was a net left to right shunt (Qp:Qs =2.4).
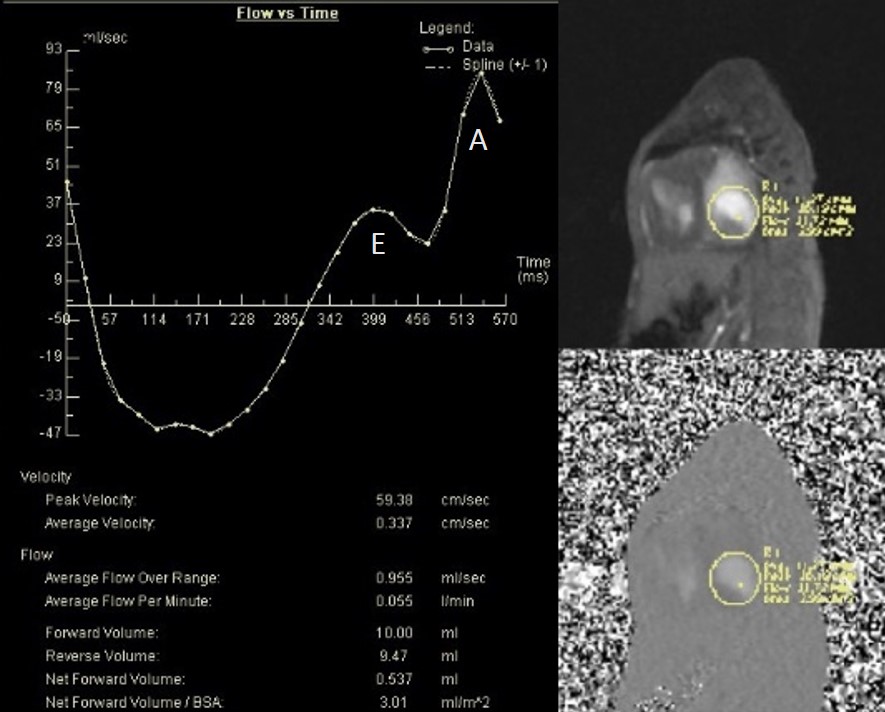
Image 4: Flow sequence across the mitral valve.
The right ventricle was significantly dilated (RVEDVi = 125.8 mL/sq.m; Z score +11) with preserved global systolic function (RVEF 56%) and mild tricuspid regurgitation [1]. The left ventricle was severely dilated (LVEDVi = 173.5 mL/sq.m; Z score +16) with extremely thin anterior, inferior and lateral walls with moderately depressed global systolic function (LVEF 43%) (Video 3).
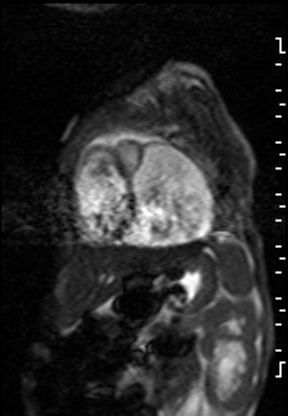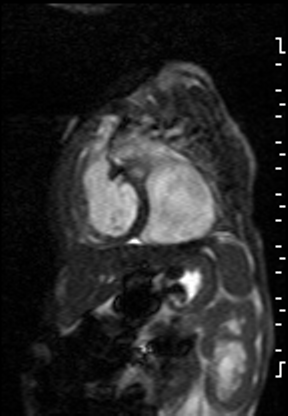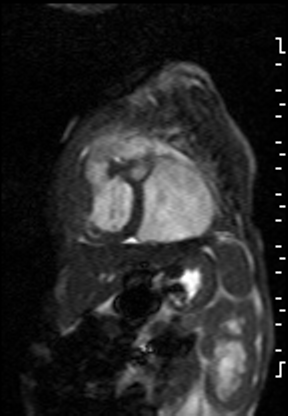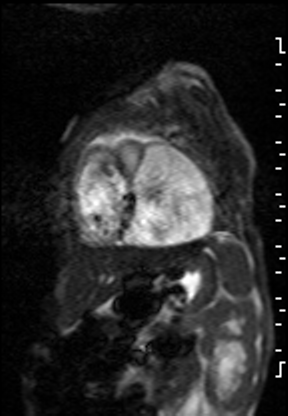
Video 3: Short axis SSFP cine demonstrating severe LV dilation with extremely thin anterior, inferior and lateral walls. Note the LV moves in synchrony with the RV but with moderate global hypokinesia.
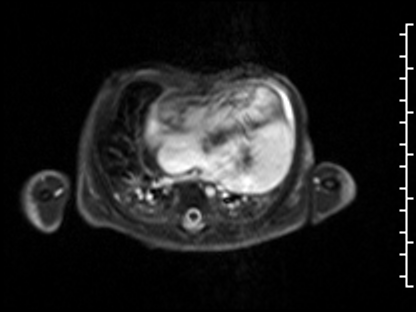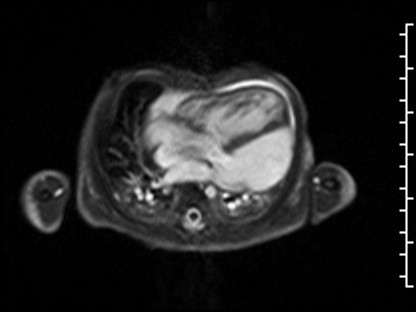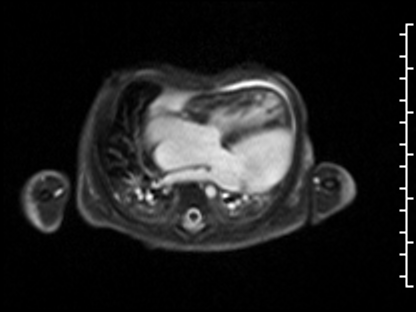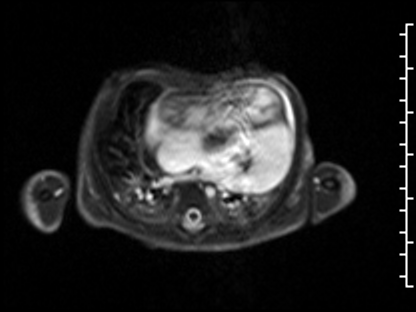
The anteroseptal and inferoseptal segments of the left ventricular wall had usual thickness and the basal anteroseptal segment connected to the outlet VSD. Following administration of gadolinium contrast, free breathing single shot late gadolinium enhancement imaging from an axial projection demonstrated transmural hyperenhancement in the lateral wall of the LV suggestive of diffuse fibrosis (Image 5).

Image 5: Delayed enhancement, 4-chamber projection. Fibrosis delineated by arrows.
Perspective: Unguarded mitral orifice (UMO) is an exceedingly rare congenital cardiac anomaly characterized by absence of mitral valve leaflets, chordae and papillary muscles at the mitral annulus with severe thinning of the left ventricular (LV) free wall [2,3]. Being devoid of any mitral tissue leads to unrestricted to-and-fro flow across the left atrioventricular junction.
The etiology of UMO remains unclear. Embryologically, development of the mitral valve takes place between the fourth and fifth weeks of gestation. Mitral leaflets, chordae tendineae and papillary muscles form by undermining of the endomyocardial aspect of the LV inlet which occurs along with immersion of the atrioventricular sulcus [4]. Yasukochi et al. hypothesized that anomaly in undermining of the endomyocardial aspect of the LV may occur either by maldevelopment or apoptosis prior to leaflets separation from the ventricular myocardium [2]. This can lead to failure of delamination of valvular leaflets and thinning of ventricular wall, the end point of which is UMO with absence of the LV parietal myocardial layer. Another hypothesis by Howley et al. proposed that a defect in normal mitral valve development may result in valve deformity leading to early development of mitral valve incompetence that could alter normal LV development resulting in thinning and dilation [5].
The few reported cases of unguarded mitral valve orifice in the literature are summarized in Table 1 [2-11]. Six cases had DORV and pulmonary atresia/stenosis, one DORV and interrupted aortic arch, one transposition and pulmonary atresia, one AV/VA concordance with hypoplastic left heart syndrome and aortic atresia, and one with AV/VA concordance and aortic atresia. Seven out of eleven cases did not survive, two with unknown outcome and 2 underwent single ventricle pathway. All reported patients were diagnosed by echocardiography postnatally except one diagnosed prenatally by fetal echocardiogram.
We report this unique case of congenital UMO in the setting of viscero-atrial situs solitus, AV concordance and DORV. Unlike other reported cases, the great arteries were normally related without aortic or pulmonary stenosis. The LV was severely dilated with extremely thin free walls and moderately depressed LV global systolic function. In our case, it was challenging to arrive at a definitive diagnosis by echocardiography and CMR was essential to establish a complete, final diagnosis. Our findings of diffuse fibrosis in the LV free wall has been demonstrated on previous autopsies [8]. To our knowledge, this is the first case of UMO that is reported with CMR images. Due to the patient’s clinical conditions, comfort care was provided few weeks after diagnosis. An autopsy was not performed.
Table 1: Summary of published cases of unguarded mitral orifice.
| Author (reference) | Year of case report | Age at diagnosis | Initial presentation | Cardiac Diagnosis | Management | Outcome |
| Ba-Atiyah et al. | 2022 | 3 months | Respiratory distress | AV concordance, DORV, normally related great arteries, no aortic/pulmonary stenosis | Comfort care | Died at 4 months |
| Howley et. al.[5]
| 2021 | Diagnosed prenatally | Respiratory distress, cyanosis and acidosis directly after birth. Atrial flutter | Concordant AV and VA connections with aortic atresia
| ECMO with bilateral pulmonary artery band | Died at day 26 of life.
|
| Banerji et. al. [11] | 2020 | Diagnosed after birth | Clinically stable after birth | Dextrocardia, discordance AV connections, DORV with pulmonary atresia. | Proposed a univentricular strategy but parents declined intervention | Unknown |
| Subramanian et al. [7] | 2019 | Postnatally | Cyanosis and respiratory distress | AV concordance, DORV, interrupted aortic arch | Comfort care | Died at home |
| Kishi et. al. [8] | 2017 | Diagnosed after birth | severe hypoxia and bradycardia | Asplenia, DORV, dysplastic tricuspid valve, and pulmonary stenosis. | Supportive
| Died on the second day of life |
| Shati, et al. [9] | 2015 | Diagnosed after birth | supraventricular tachycardia
| Concordance AV and discordance VA, and pulmonary atresia. | Surgical shunt aiming for single ventricle approach | Died postoperatively |
| Su, et. al. [10] | 2014 | Diagnosed after birth | Cyanosis | Concordant AV and VA connection, hypoplastic left heart syndrome and aortic atresia. | Atrial septectomy and bilateral pulmonary artery banding on day 1 of life. Norwood procedure with Sano modification day 5of life. | Unknown |
| Hwang et. al. [6] | 2010 | Diagnosed after birth | Cyanosis | Discordance AV connections, DORV with pulmonary atresia. | Modified left Blalock–Taussig shunt at 33 days old | Single ventricle pathway |
| Earing et. al. [3]
| 2003 | 5 days old
| Cyanosis | Discordance AV connections, DORV with pulmonary atresia. | Mee operation at 7 days old. bidirectional Glenn shunt at 6 months old | Single ventricle pathway |
| Yasukochi et. al. [2]
| 1999 | 9 months old | Cyanosis, CHF | Discordance AV connections, DORV with pulmonary stenosis. | At 15 months of age, left Blalock-Taussig shunt. | Died of CHF and ventricular fibrillation at 17 years old.
|
| Yasukochi et. al. [2] | 1999 | 9 days old | Cyanosis | Discordance AV connections, DORV with pulmonary stenosis. | Left Blalock–Taussig shunt at 23 days old
| Died of CHF and ventricular fibrillation at 13 months old
|
AV: Atrioventricular, VA: Ventriculoarterial, ECMO: extracorporeal membrane oxygenation, DORV: double-outlet right ventricle, CHF : congestive heart failure
Click here to view the entire study on CloudCMR: https://www.cloudcmr.com/6457-1973-0508-0124/
References
- Buechel EV, Kaiser T, Jackson C, et al. J Cardiovasc Magn Reson. 2009 Jun 21;11:19.
- Yasukochi S, Satomi G, Park I, Ando M, Momma K. Unguarded mitral orifice mirror-imaged atrial arrangement and discordant atrioventricular connections. Cardiology in the Young. 1999;9:478-483.
- Earing MG, Edwards WD, Puga FJ, Cabalka FK. Unguarded mitral orifice associated with discordant atrioventricular connection double-outlet right ventricle and pulmonary atresia. Pediatric Cardiology. 2003;24:490-492.
- Wenink ACG, Gittenberger-de Groot AC, Brom AG. Developmental considerations of mitral valve anomalies. Int J Cardiol 1986;11:85–98.
- Howley LW, Strasburger J, Maleszewski JJ, Snowise S, Lund A, Schneider A, MacIver R, Edens E, Eyerly-Webb S, Silverman NH. Fetal unguarded mitral valve orifice aortic atresia and severe left heart enlargement. JACC Case Rep. 2021;3:206-211.
- Hwang MS, Chang YS, Chu JJ, Lin WS, Su WJ. A potential new constellation of defects: unguarded mitral orifice associated with double-outlet right ventricle {I,D,D} and pulmonary atresia/stenosis. Int J Cardiol. 2011;148:354-357.
- Subramanian, et al. Unguarded left atrioventricular orifice: An unusual cause of hypoplastic left ventricle and double-outlet right ventricle with intact ventricular septum.” Ann Pediatr Cardiol. 2019;12(2):153-155.
- Kishi K, Katayama H, Ozaki N, Odanaka Y, Masuda M, Nemoto S, Satomi H, Okada Y, Tamai H. Fatal cardiac anomaly of unguarded mitral orifice with asplenia syndrome. J Cardiol Cases. 2016;15:6-9.
- Ayed S, et al. A female neonate with unguarded mitral valve orifice. Journal of the Saudi Heart Association. 2015;27(4): 324.
- Su JA, Ho J, Wong PC. Unguarded mitral orifice associated with hypoplastic left heart syndrome. Cardiol Young. 2015:25:1002-1005.
- Banerji N, Krishna MR, Kumar RK, Anderson RH. Caught-off guard: unguarded mitral valve orifice in usual atrial arrangement with discordant atrioventricular connections and pulmonary atresia. Ann Pediatr Cardiol. 2020;13:84.
Case prepared by:
Robert D. Tunks, MD, MHS
Associate Editor, SCMR Case of the Week
Penn State Health, Milton S. Hershey Medical Center
Hershey, PA USA





