Cecilia Kwok BMSc MSc MD1, Han S. Lim MBBS FRACP PhD2, Mark Nolan MBBS FRACP PhD1,3
1. Cardiology Department, Western Health, St Albans, Victoria, Australia
2. Cardiology Department, Austin and Northern Health, University of Melbourne, Victoria, Australia
3. Baker Heart and Diabetes Institute, Melbourne, Australia
Clinical History
A 60 year-old woman was referred to cardiology clinic to investigate asymptomatic ECG abnormalities detected during workup for elective hemicolectomy for malignancy. Her medical history included rheumatoid arthritis, obstructive sleep apnoea, osteoarthritis and obesity. Her younger half-brother died in his 50’s from sudden cardiac death whilst playing sports. Medications included metoprolol XL and warfarin. Physical examination was unremarkable. Her electrocardiogram demonstrated rightward-axis deviation, right-bundle-branch-block, T-wave inversion in leads V1 to V4 and small positive deflections at the end of the ECG in leads V3 to V5 (Figure 1). Her echocardiogram demonstrated severe asymmetrical left ventricular (LV) wall thickening, akinesis of all apical segments, and a mass in LV apex (Movies 1-2). Coronary angiography showed no significant coronary artery disease (Figure 2). She was referred for a cardiovascular magnetic resonance (CMR) to further investigate her cardiomyopathy and suspected LV apical thrombus.
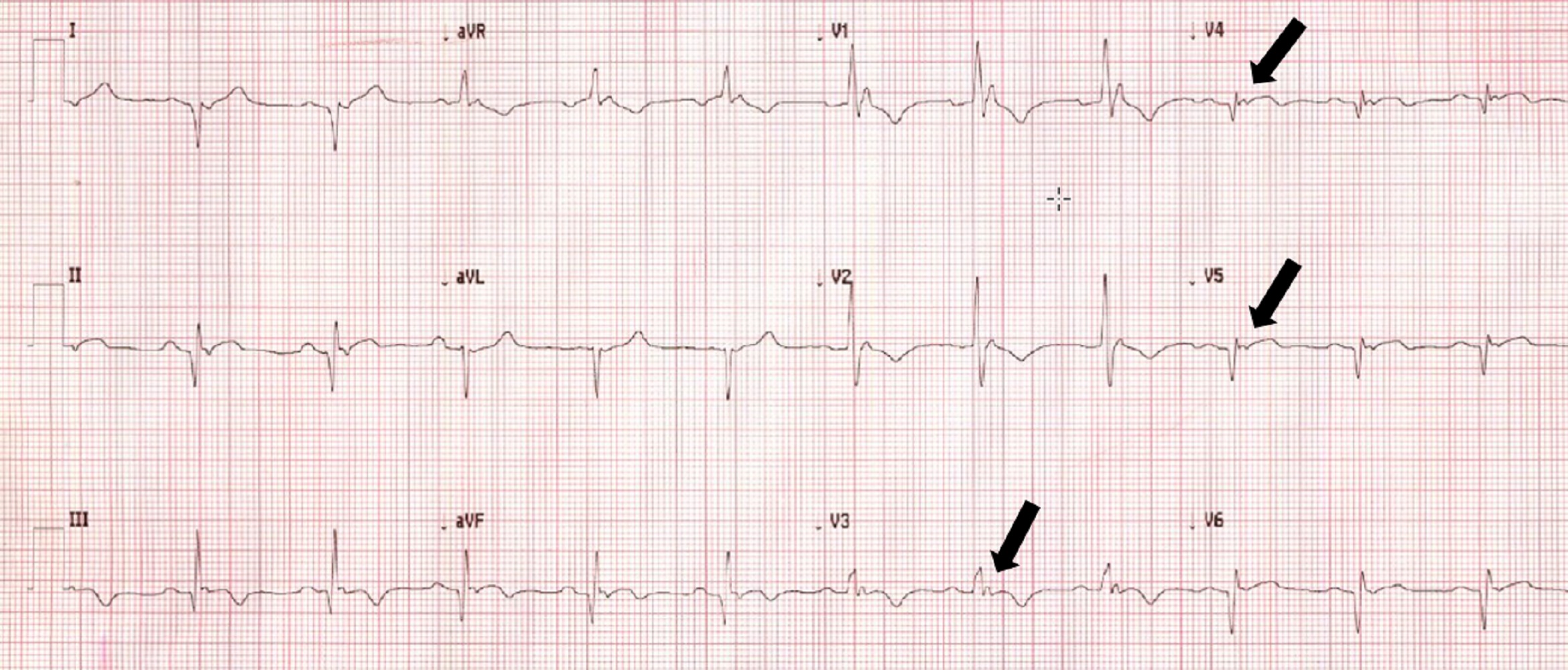
Figure 1: Twelve lead ECG demonstrating right-ward axis with right bundle branch block and epsilon waves in leads V3 to V5.

Movie 1 and 2: Transthoracic echocardiogram 4 chamber without and with Definity contrast enhancement demonstrating asymmetrical severely increased left ventricular wall thickness, akinesia of apical segments, and small hypointense mass in the apex.

Figure 2: Images from invasive coronary angiography demonstrating normal left-sided coronary arteries (A) and normal right coronary artery (B).
CMR Findings
Study was performed on 1.5T system with sinus rhythm of 72 bpm. Cine imaging demonstrated mildly dilated LV of 141 mL and normal LV ejection fraction of 55% (Movie 3). Aneurysmal dilatation of LV apical segments was present with 17 x 13 mm thrombus (Movie 4). There was no evidence of non-compaction. Increased LV wall thickness was seen in the basal-to-mid septal segments with maximal thickness of 21 mm (Movie 5). Papillary muscles demonstrated normal structure and attachments to LV walls. Systolic anterior motion of mitral leaflets was present. Right ventricle (RV) was dilated with end-diastolic volume of 240 mL and RV ejection fraction of 27% (Movie 6). “Accordion” sign was present in subtricuspid RV free wall and associated with dyskinesis and microaneurysmal changes (Movies 6-7). The “accordion sign” has been described in the literature as focal crinkling of the RV free wall, typically in the sub-tricuspid or the right ventricular outflow tract region, that is caused by multiple sequential outpouching that do not reach microaneurysmal size (1). Parametric mapping was not available. Delayed enhancement imaging demonstrated patchy mid-wall scar in the hypertrophied LV segments but no obvious confluent scar was seen in LV apex (Figure 3). Myocardial scar comprised 14% of total LV myocardium. There was subtle delayed enhancement in the sub-tricuspid region of the RV free wall (Figure 4).
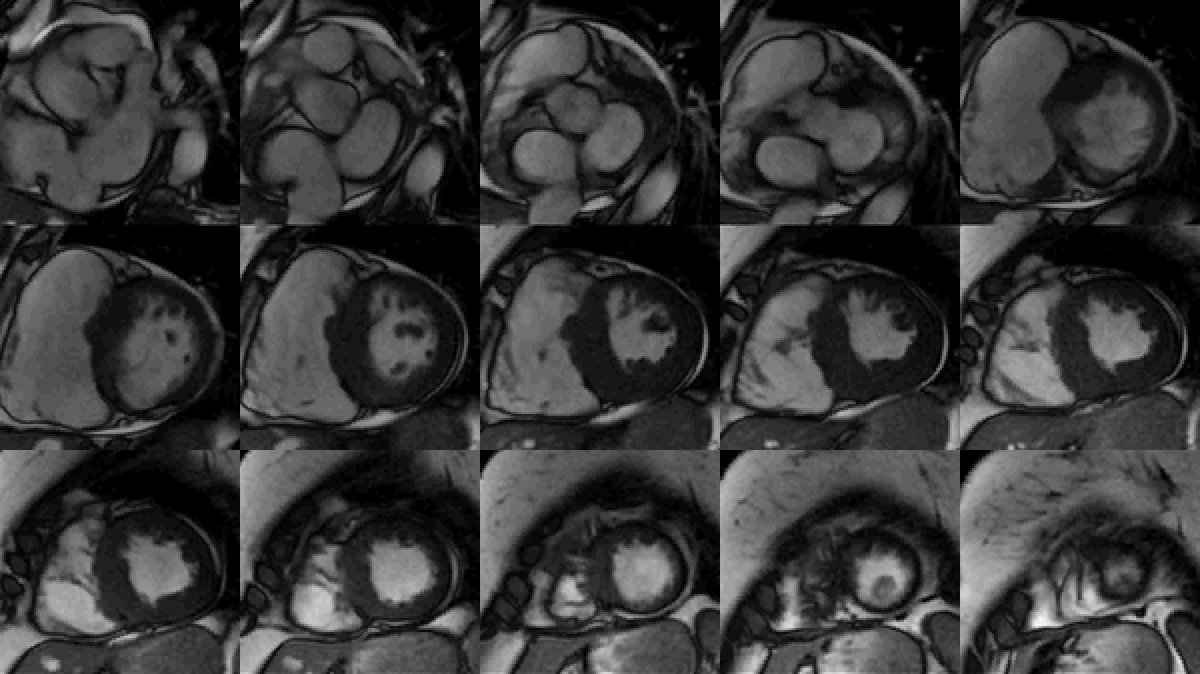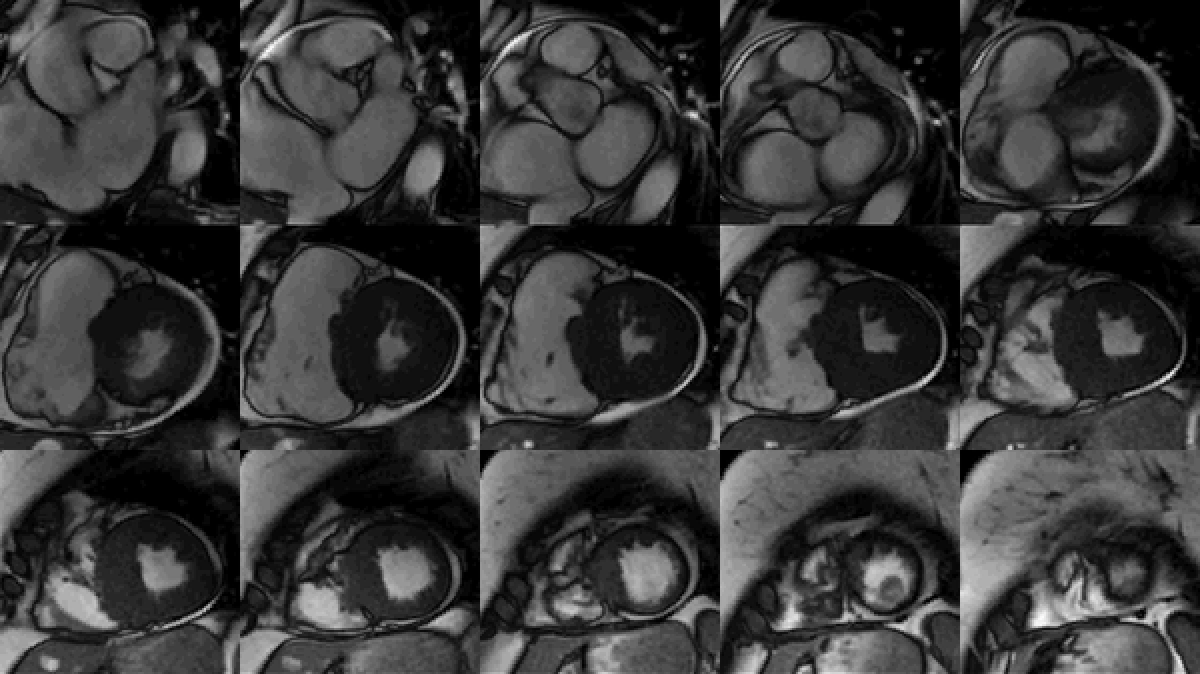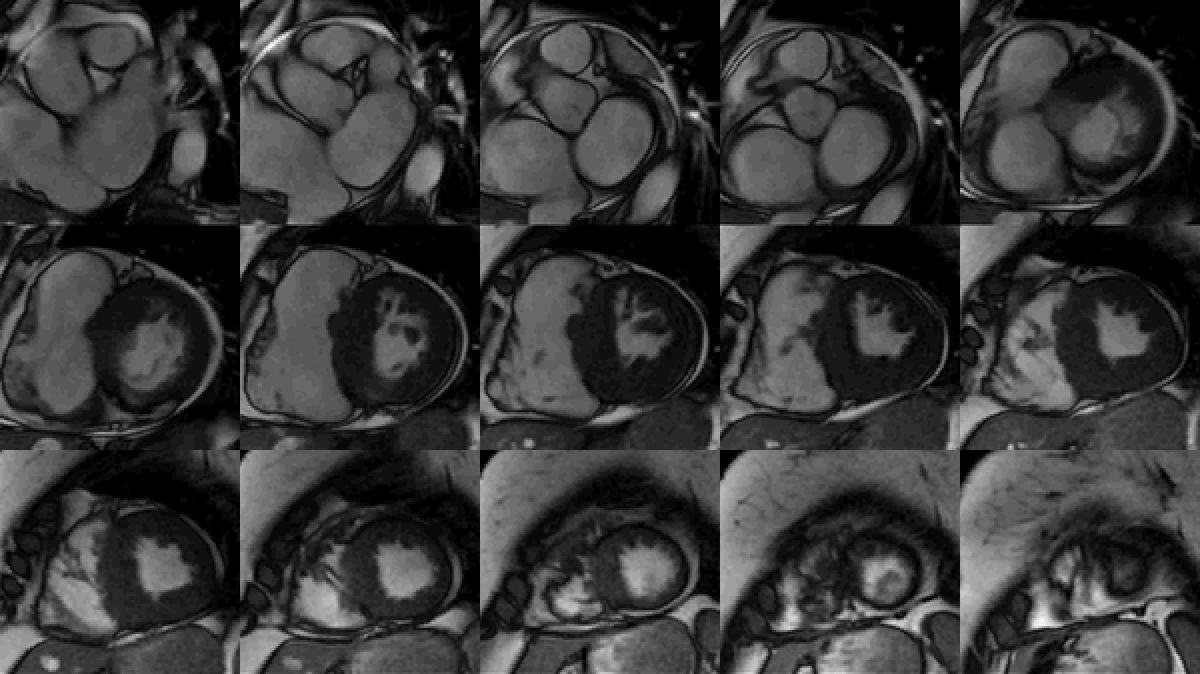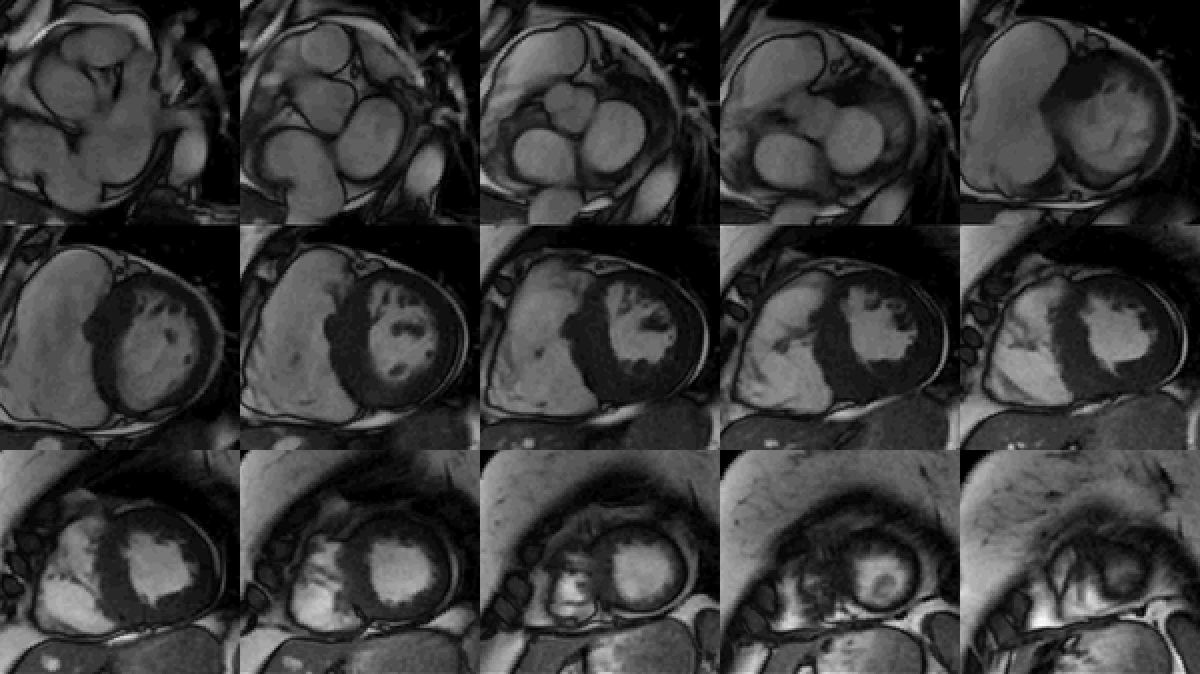
Movie 3: SSFP cine short-axis stack shows asymmetrically increased left ventricular (LV) wall thickness (maximum diameter 21 mm) of basal-to-mid septal and anterior segments with LV apical aneurysm and probable thrombus. Small pericardial effusion.

Movie 4: SSFP cine two chamber view demonstrating severe asymmetrical increased left ventricular (LV) wall thickness. Probable thrombus seen in the LV apex.

Movie 5: SSFP cine imaging of left ventricular three-chamber view demonstrating increased septal wall thickness with systolic anterior motion of mitral leaflets and dynamic left ventricular outflow tract obstruction. Dyskinetic systolic bulging of a microaneurysm can be seen in the right ventricular outflow tract free wall.

Movie 6: SSFP cine four-chamber view demonstrating dilated right ventricle (RV) with “accordion sign” in sub-tricuspid “triangle of dysplasia”. Note that small outpouching of apical RV free wall is due to tethering by moderator band and is a normal variant that may be mistaken for regional wall motion abnormality.
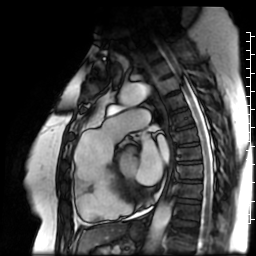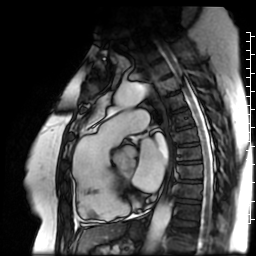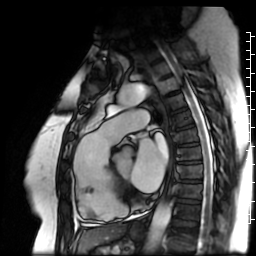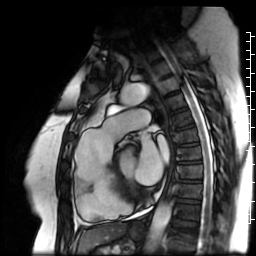
Movie 7: SSFP cine right ventricular outflow tract (RVOT) view demonstrating akinesia of the RVOT free wall and dyskinetic aneurysm in right ventricular inferior wall.

Figure 3: Delayed gadolinium imaging of two-chambered view demonstrates mid-wall patchy myocardial scar can be seen in left ventricular inferior wall and is consistent with hypertrophic cardiomyopathy.
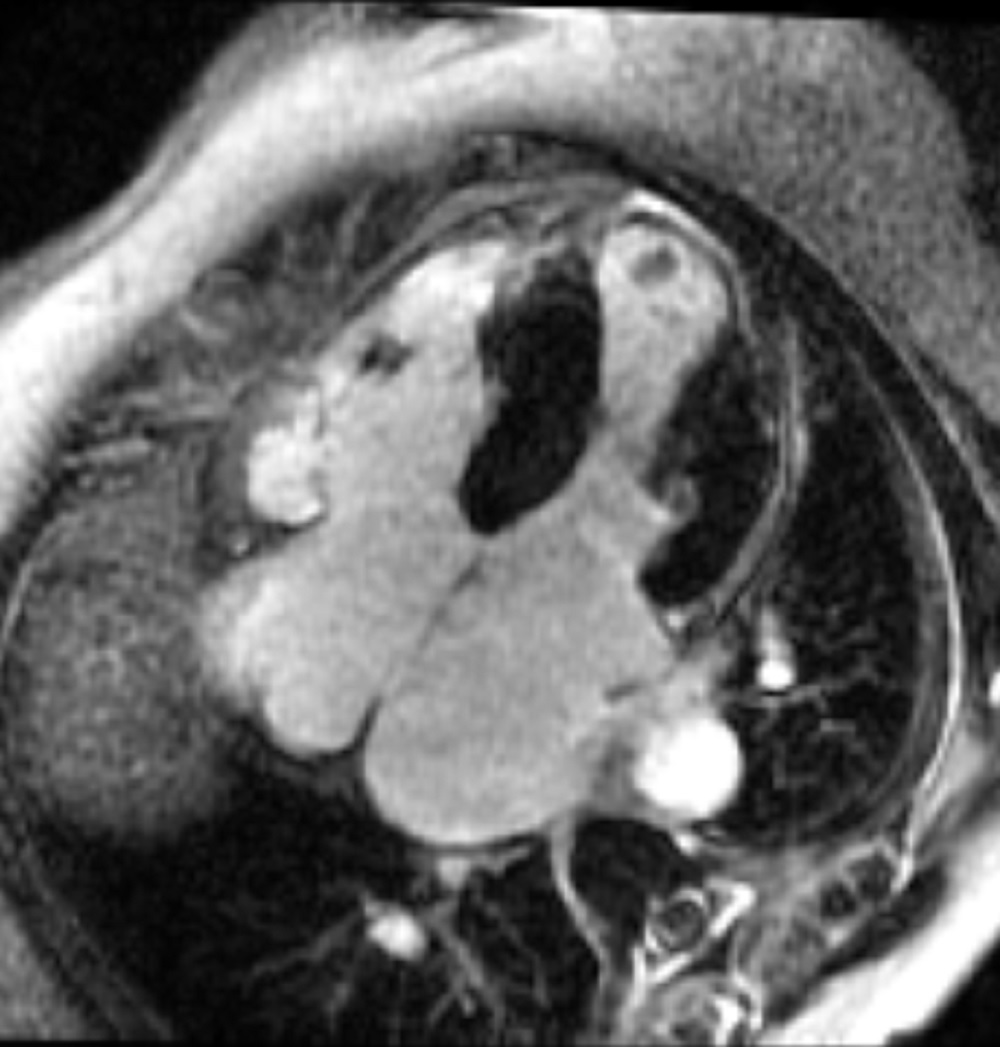
Figure 4: Delayed gadolinium imaging of four-chamber view shows left ventricular aneurysm without confluent myocardial scarring. Presence of thrombus is confirmed by avascularity of mass. There is subtle delayed enhancement in the sub-tricuspid region of right ventricular free wall which is part of the “triangle of dysplasia” in arrhythmogenic cardiomyopathy.
Conclusion
Genetic analysis revealed a pathogenetic mutation in the Plakophillin 2 (PKP2) gene and a strong class 3A variant of uncertain significance in the FHOD3 gene, which is a gene associated with hypertrophic cardiomyopathy (2). Final diagnosis was concomitant genetic cardiomyopathies of arrhythmogenic cardiomyopathy (AC) and hypertrophic cardiomyopathy (HCM) due to digenic inheritance complicated by large left ventricular apical aneurysm and thrombus.
Perspective
Based on CMR findings, patient underwent further investigations. Electrophysiology review of ECGs confirmed presence of epsilon waves (Figure 1, arrows). Her warfarin was ceased one month prior to her CMR based on contrast-enhanced echocardiogram report that suggested absence of thrombus. Warfarin was subsequently restarted 3 weeks after her CMR study; however, one week after that she suffered a stroke with large frontoparietal and basal ganglia infarction demonstrated on brain MRI. Her INR was subtherapeutic (INR 1.3) at time of stroke. She underwent prolonged rehabilitation course with excellent recovery. Family members were provided with an anonymized letter offering genetic counselling and cardiac screening every 3-5 years from childhood was recommended. Holter monitor showed sinus rhythm throughout with 0.5% burden of ventricular ectopics. From the arrhythmia specialist perspective, due to the patient being reluctant to consider an implantable cardioverter defibrillator (ICD) for primary prevention, the decision for a loop recorder with close rhythm monitoring was made with the option to upgrade to ICD implantation if further high-risk features were identified.
AC diagnosis is supported by ECG, imaging and genetic findings and meets 2010 Revised Taskforce Criteria for diagnosis of AC (3). Although it may be tempting to attribute both cardiomyopathic phenotypes to a single pathogenic gene variant, PKP2 pathogenic variants to date have not been associated with hypertrophic phenotypes (4). It is possible that cycles of temporarily ceasing and restarting anticoagulation based on alternating imaging modalities with differing sensitivities for thrombus may have contributed to thrombus instability and consequent stroke.
This case demonstrates coexistence of two separate cardiomyopathic phenotypes that is likely attributable to digenic inheritance and the presence of pathogenic variants in two different cardiomyopathy genes. Very little published literature is available regarding prevalence of co-existing cardiomyopathies.
If pathologic variants are inherited independently and assuming genetic cardiomyopathy prevalence of 0.2%, then it might be expected that 1 in 2,500 individuals could have ≥2 pathogenic cardiac mutations. Features supporting causal role of VUS in FHOD3 gene for HCM phenotype in this case include rarity of variant, in silico computational simulation predicts pathogenicity and comparable VUS examples display moderate pathogenicity. Further research in this clinical is needed. Echocardiographic imaging correctly identified presence of HCM but likely did not detect presence of persisting left thrombus presence and missed diagnostic imaging criteria for AC.
This case also highlights the central role of CMR in investigation and diagnosis of RV pathology. Indeed, it was only in the context of CMR findings that several relevant pre-existing features such as epsilon waves on ECG were finally recognized. Prior studies have shown echocardiography can only identify 50% of AC cases that are identified by CMR, which suggests that selective use of CMR may double number of identified cases of this life-threatening disease (5, 6). This case supports the concept that lowering testing threshold and improving access to advanced cardiac imaging can improve patient outcomes.
It should be recognized that despite accurate pathological diagnosis, CMR did not prevent patient from a potentially avoidable adverse outcome within days of testing. Warfarin was apparently ceased on basis of a negative contrasted-enhanced echocardiogram – however accuracy of contrast-enhanced echo compared to LGE-CMR is only 61% and it is likely this echo finding was a false-negative given subsequent CMR findings (7).
Patient’s use of hydroxychloroquine briefly raised the possibility of hydroxychloroquine cardiotoxicity, which is a recognized phenocopy of HCM (8). However several features of this case made hydroxychloroquine less likely including absence of bradycardia, presence of potentially pathological mutation in gene associated with HCM, presence of asymmetrical hypertrophy and presence of systolic anterior mitral motion and of apical aneurysm (8). If hydroxychloroquine is continued then it may be reasonable to repeat CMR with T1 mapping as lysosomal storage dysfunction due to hydroxychloroquine lowers T1 mapping times and perhaps could be confirmed with endomyocardial biopsy.
We wish to thank Dr. Simon Bodek, Clinical Geneticist at Austin Health in Melbourne, Australia for his valuable contribution.
Click here to view the entire study on CloudCMR.
References
1.Dalal D, Tandri H, Judge DP, Amat N, Macedo R, Jain R, et al. Morphologic variants of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy a genetics-magnetic resonance imaging correlation study. Journal of the American College of Cardiology. 2009;53(15):1289-99.
2.Ochoa JP, Sabater-Molina M, Garcia-Pinilla JM, Mogensen J, Restrepo-Cordoba A, Palomino-Doza J, et al. Formin Homology 2 Domain Containing 3 (FHOD3) Is a Genetic Basis for Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2018;72(20):2457-67.
3.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806-14.
4.Christensen AH, Benn M, Bundgaard H, Tybjaerg-Hansen A, Haunso S, Svendsen JH. Wide spectrum of desmosomal mutations in Danish patients with arrhythmogenic right ventricular cardiomyopathy. J Med Genet. 2010;47(11):736-44.
5.Borgquist R, Haugaa KH, Gilljam T, Bundgaard H, Hansen J, Eschen O, et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria. Eur Heart J Cardiovasc Imaging. 2014;15(11):1219-25.
6.Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O, et al. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy-an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18(3):237-53.
7.Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovascular imaging. 2009;2(8):969-79.
8.Ezzeddine FM, Giudicessi JR, Maleszewski JJ, Lin PT, Borlaug BA, Geske JB. Unmasking Hydroxychloroquine Cardiotoxicity in a Patient With Heart Failure and Chronotropic Incompetence. JACC Case Rep. 2021;3(7):997-1001.
Case prepared by:
Jason N. Johnson, MD MHS
Deputy Editor, Cases of SCMR
Le Bonheur Children’s Hospital, University of Tennessee Health Science Center, St. Jude Children’s Research Hospital







