Rimmy Garg MD, Carlos Requena Armas MD, Vijayasree Paleru MD
University of Illinois College of Medicine Peoria, OSF St. Francis Medical Center, Peoria, Illinois
Clinical History
A 62-year-old female with a past medical history of hypertension and hyperlipidemia presented with recurrent episodes of sudden onset, mid-sternal chest pain radiating to the neck and upper back while at rest for the past day. On physical exam, the patient was noted to be hypertensive to 187/98 mmHg and tachycardic to 103 bpm with no other remarkable finding. Serial high-sensitivity troponins trended upward from < 4 ng/L to 23,121 ng/L (normal <= 17 ng/L). Other lab values were within normal range. A 12-lead ECG demonstrated an incomplete right bundle branch block with T-wave inversions in the anterior leads [Figure 1].
Due to concern for a non-ST elevation myocardial infarction (NSTEMI), the patient was taken for coronary angiography, which revealed tapering of the superior branch of the obtuse marginal 1 (OM1) artery, concerning for spontaneous coronary artery dissection (SCAD) [Figure 2, Video 1]. There was no other evidence of obstructive coronary artery disease (CAD). Left ventriculogram from right anterior oblique angle demonstrated severe hypokinesis of the mid-anterior wall [Video 2]. Transthoracic echocardiogram, (TTE) revealed a left ventricular ejection fraction (LVEF) of 55% with focal area of moderate hypokinesis at the mid to distal inferolateral [Video 3] and anterolateral walls [Video 4]. LV end-diastolic volume was 61.5ml (indexed to 34m2) and LV end-systolic volume was 22.8ml (indexed to 13m2).
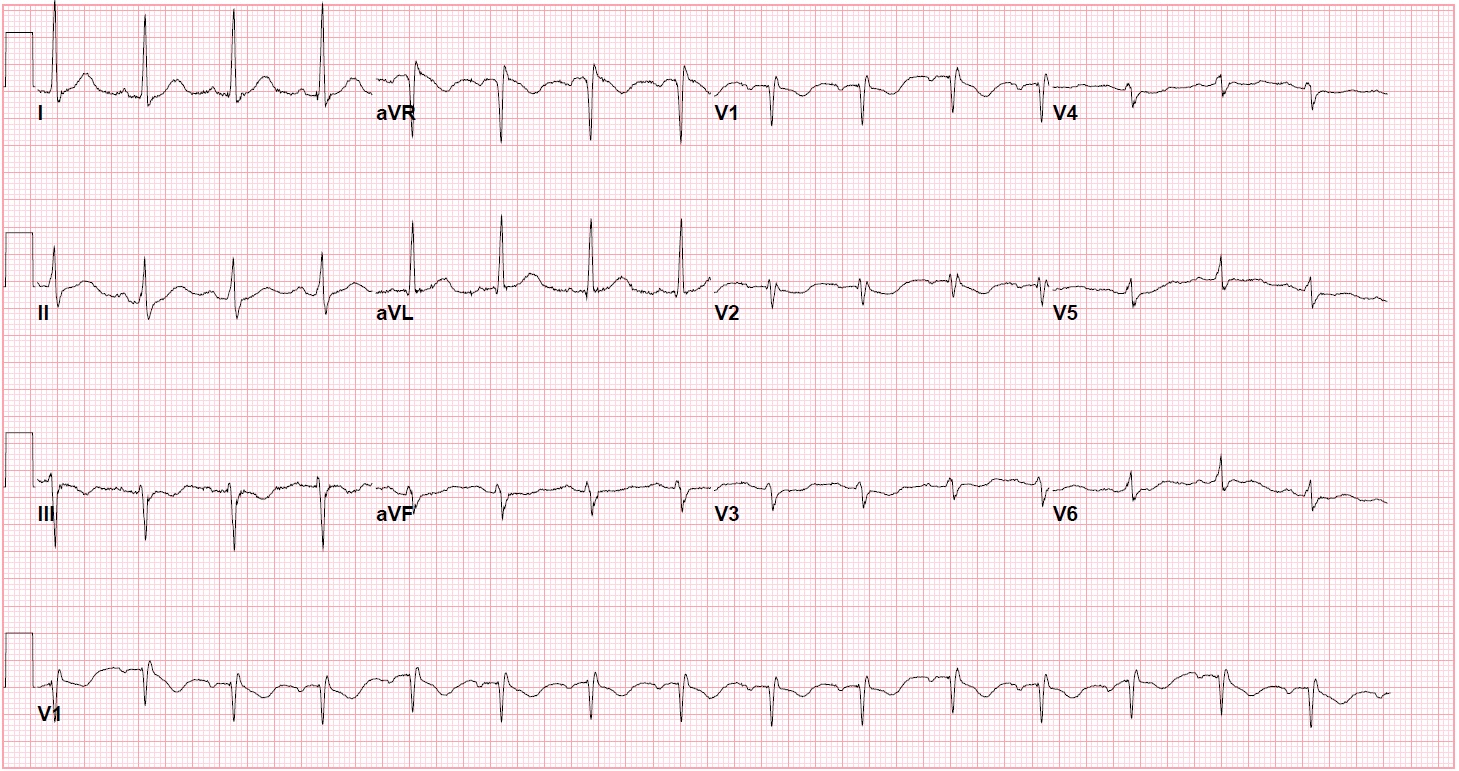
Figure 1: Presenting ECG with an incomplete right bundle branch block with T-wave inversions in the anterior leads.

Figure 2 and Video 1. Coronary angiography demonstrating an abrupt tapering (arrow) of the obtuse marginal 1 branch of the left circumflex, concerning for spontaneous coronary artery dissection.
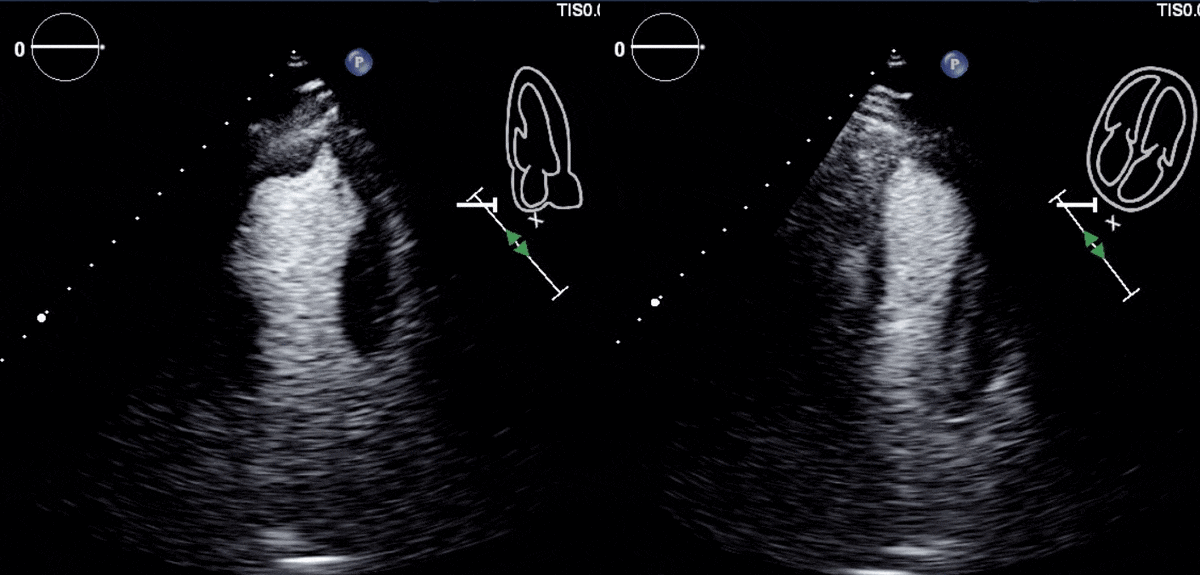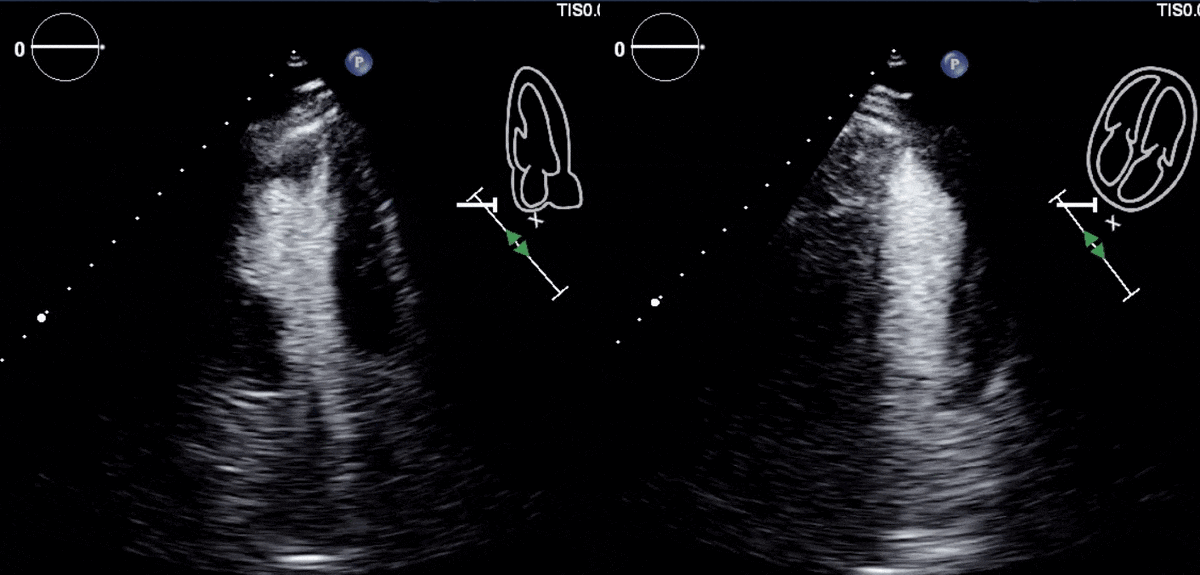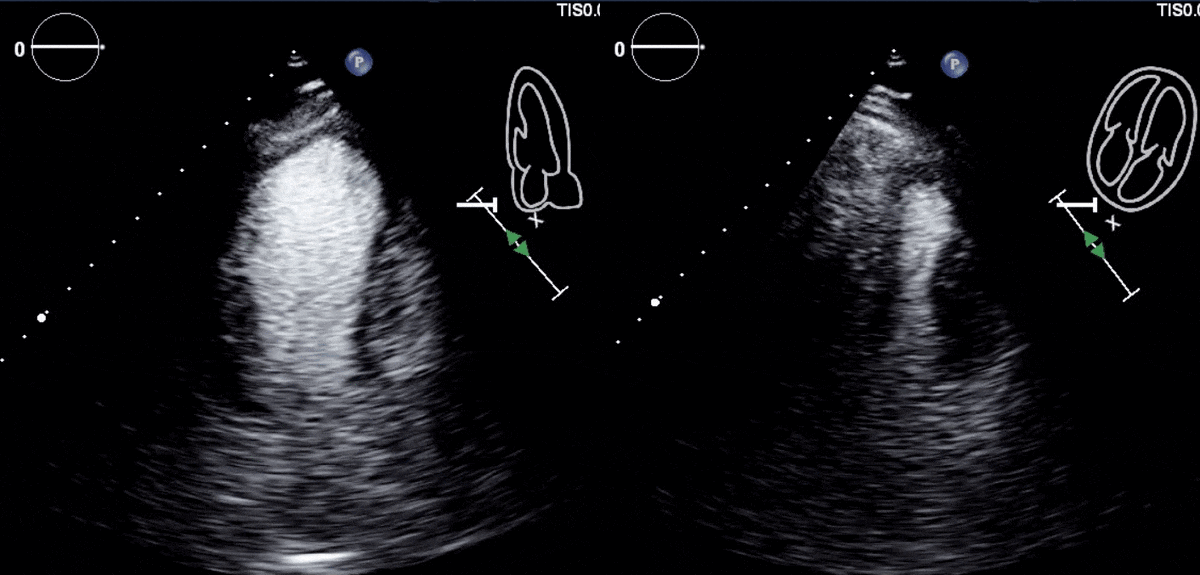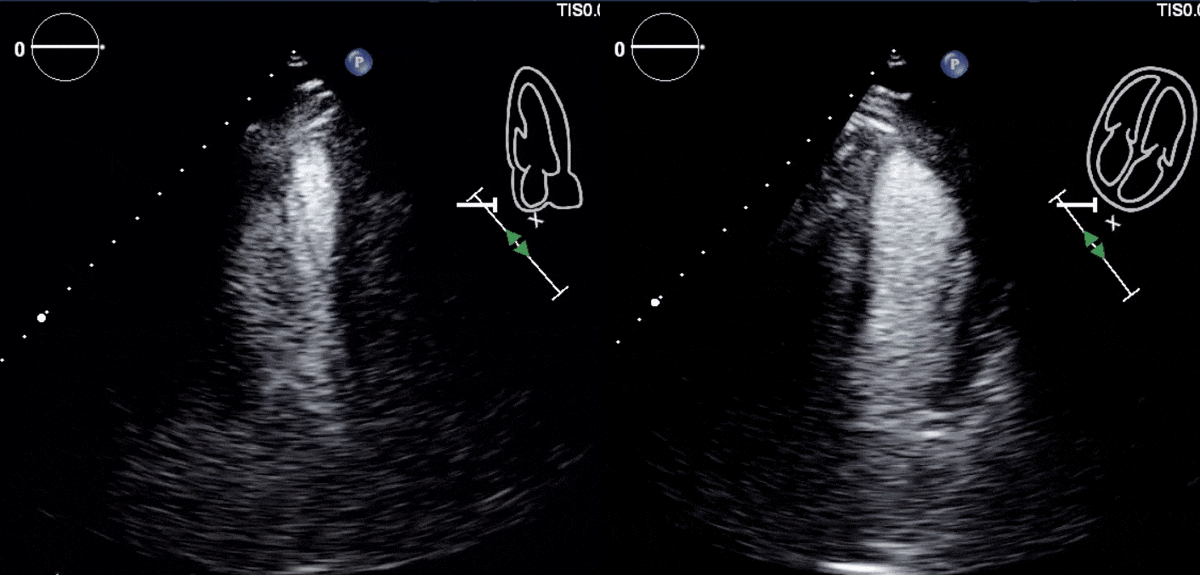
Video 3 and 4: TTE with contrast agent demonstrating hypokinesis of the mid to distal infero-lateral and antero-lateral walls.
CMR Findings:
As the area of regional wall motion abnormality did not correlate with the coronary distribution of the SCAD vessel and there was discrepancy between the wall motion abnormality noted on the coronary angiogram and that noted on the TTE, further evaluation was performed using a cardiovascular magnetic resonance (CMR) study. CMR imaging with and without contrast was performed on a 1.5 T GE Optima MR 450w scanner. Cine imaging using the FIESTA sequences was used to evaluate LV morphology and function. Tissue imaging was performed using double and triple inversion recovery sequences. Standard first pass perfusion and delayed gadolinium enhancement (DGE) imaging was performed.
The patient was found to have a normal left ventricular size with an LVEF of 51% with focal mid to distal inferolateral and anterolateral wall severe hypokinesis, sparing the apex [Video 5-6]. Also in this area, there was edema [Figure 3A-B] with subendocardial to near transmural delayed enhancement. Lateral subendocardial perfusion defect was also noted on resting first pass perfusion imaging [Video 7 and Figure 4]. There is evidence of a small area of microvascular obstruction (MVO) as well [Figure 5A-B]. Patient otherwise had normal size and systolic function of the right ventricle with a normal pericardium.

Video 5 and 6. SSFP/FIESTA Cine Images in four chamber and short axis views showing focal akinesia in the lateral apical segment.
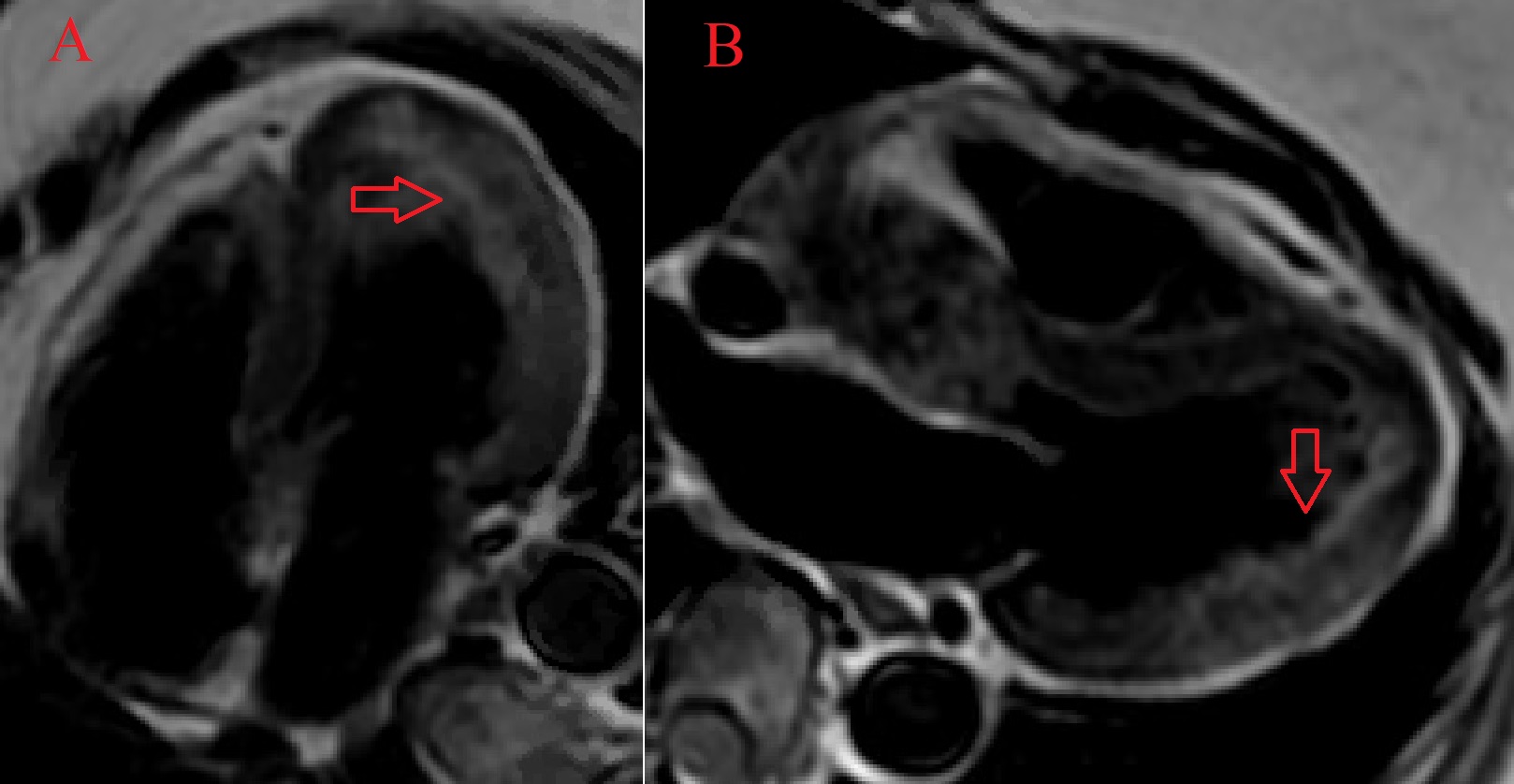
Figure 3. CMR double inversion recovery sequence in the 4-chamber view (A) and the 3-chamber view (B) demonstrating edema of the mid to distal anterolateral wall (arrows).
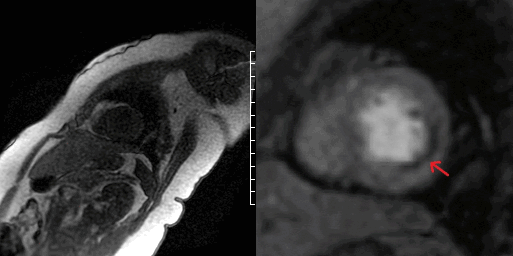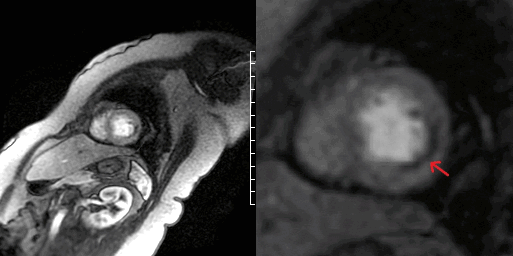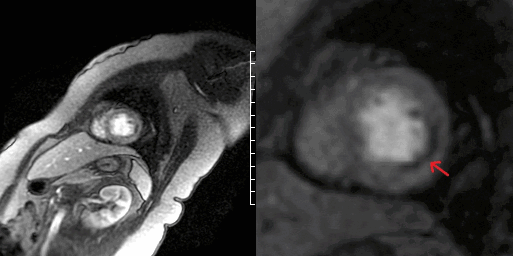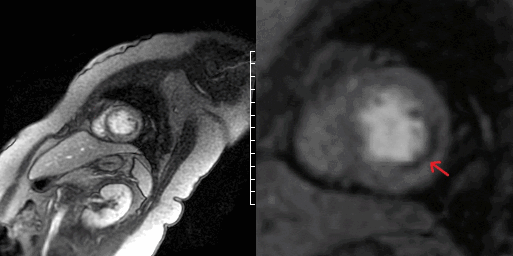
Video 7 and Figure 4. Apical segment of LV showing perfusion defect on first pass imaging (arrow).
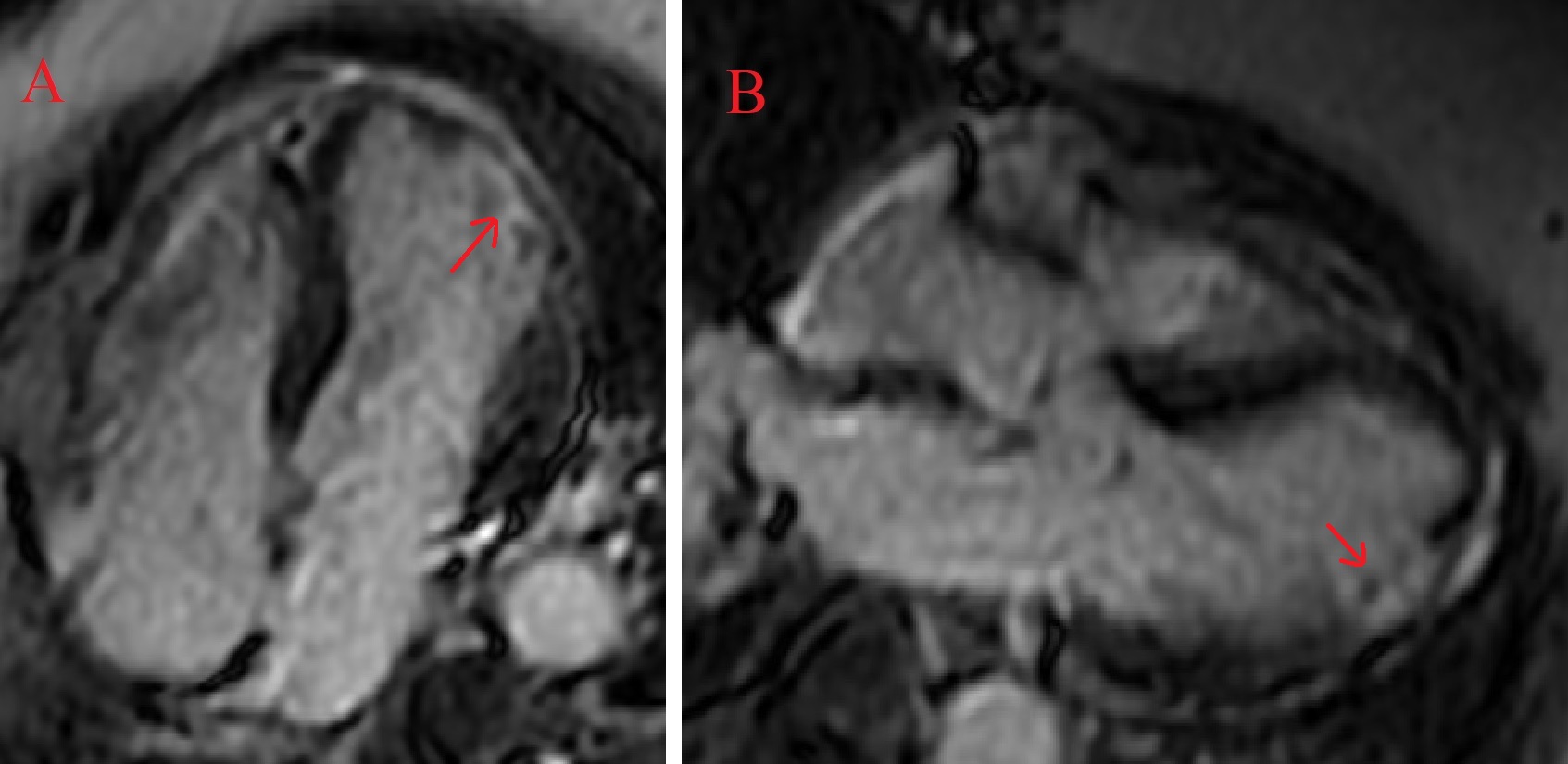
Figure 5. CMR demonstrating subendocardial delayed gadolinium enhancement with pockets of microvascular obstruction (arrows) at the anterolateral wall in a 4-chamber view (A) and the inferolateral wall in 3-chamber view (B).
Conclusion
The CMR findings were suggestive of focal, near transmural acute infarct consistent with OM1 SCAD in the setting of the clinical history and recent coronary angiogram findings. While focal myocarditis and infiltrative cardiomyopathy can also look similar on CMR, they are less likely in this scenario. Thus, the patient was managed with dual antiplatelet therapy, beta-blocker, and appropriate lipid-lowering agents. On a follow-up clinic visit, she was noted to be chest pain free without residual ischemic symptoms.
Perspective
In women, SCAD is an important cause of acute myocardial infarction and sudden cardiac arrest; however, it can often be misdiagnosed, or go undiagnosed, due to inherent limitations in current coronary angiogram techniques [1]. While use of optical coherence tomography and intravascular ultrasound can help better visualize the arterial wall, such instrumentation can lead to worsening of dissection and won’t help determine the extent of the myocardial damage [2]. Furthermore, the severity of ischemia/infarction associated with SCAD is highly variable; the majority of patients have a small-sized MI with preserved LVEF [3].
Knowledge of the pathophysiology of myocardial infarction due to epicardial occlusion is essential to the understanding of SCAD-associated CMR findings. Initially, the myocardial necrosis progresses from the sub-endocardium towards the epicardium [4]. Then, the degree of revascularization achieved spontaneously or via PCI leads to various levels of reperfusion injury that is reflected by edema and MVO. MVO is the pathologic correlation of the no-reflow phenomenon usually after delayed recanalization and has multiple causative mechanisms (primary endothelial destruction, extravascular compression from interstitial edema, vasoconstriction induced by mediators released by the culprit lesion, obstruction by embolized debris, in-situ platelet aggregation) [5].
Gadolinium is an extracellular contrast agent and persists longer in the areas of disrupted necrotic myocardial cells, allowing for measurement of the infarct size with the T1-weighted images in both early and late post-MI period. Expert consensus recommends CMR for DGE extent to be done approximately 5 days after the event to minimize confounding from the initial dynamic infarct and associated edema [6]. MVO will appear as a hypointense core within the hyperintense infarct zone within the area of DGE reflecting the failure of the contrast agent to penetrate the infarct core. These markers have been well established in the assessment of atherosclerotic myocardial infarction and only recently applied to the evaluation of SCAD cases. Two recent studies demonstrated how CMR was valuable in confirming SCAD as the cause of acute MI by demonstrating evidence of necrosis in a particular vessel territory, hence ruling out other causes like Takotsubo cardiomyopathy and myocarditis [2, 7]. In general, CMR is essential in the work-up for and diagnosis of various causes of myocardial infarction with non-obstructive coronary arteries (MINOCA) due to its superior ability to characterize the myocardium, safety, accuracy, and inter-observer consistency.
Our case adds to the list of CMR-confirmed SCAD cases and demonstrates how CMR is an important tool for the diagnosis in the setting of acute coronary syndrome-like presentation but with discrepant angiographic findings. Early and appropriate diagnosis allows for initiation of optimal medical therapy as well as for potential prognostic assessment by evaluation of microvascular obstruction.
Click here to view the entire CMR on CloudCMR.
References
Case prepared by:
Ashish Aneja
Associate Editor, Cases of SCMR
Jason N. Johnson, MD MHS
Deputy Editor, Cases of SCMR
The University of Tennessee Health Science Center, Le Bonheur Children’s Hospital, St. Jude Children’s Research Hospital







