Syncope in a young athlete
Cara Hoke, MD, Anna Lisa Chamis, MD, M. Jay Campbell, MD
Duke University Medical Center, Durham NC, USA
Clinical History:
14-year-old male without significant past medical history presented to the Emergency Department (ED) for further evaluation after a syncopal episode. He was playing basketball when he suddenly felt unwell and collapsed. He regained consciousness about 5 minutes afterward on scene. On emergency medical services arrival, heart rate was 60bpm, blood pressure 60/30mmHg. His electrocardiogram by EMS (Figure 1) demonstrated diffuse ST segment abnormalities, which improved upon arrival to ED (Figure 2). Initial laboratory findings showed elevated troponin I of 4.18 ng/L (Ref range < 0.05ng/L) and NT-pro BNP 267 pg/mL (Ref range <125pg/mL).
Of note, he had a similar syncopal event while playing basketball six months prior to his current presentation. At that time, he underwent extensive workup at an outside hospital including echocardiogram, treadmill stress testing, cardiac MRI, Holter monitoring, EP study, and genetic testing for hypertrophic cardiomyopathy which were all negative.
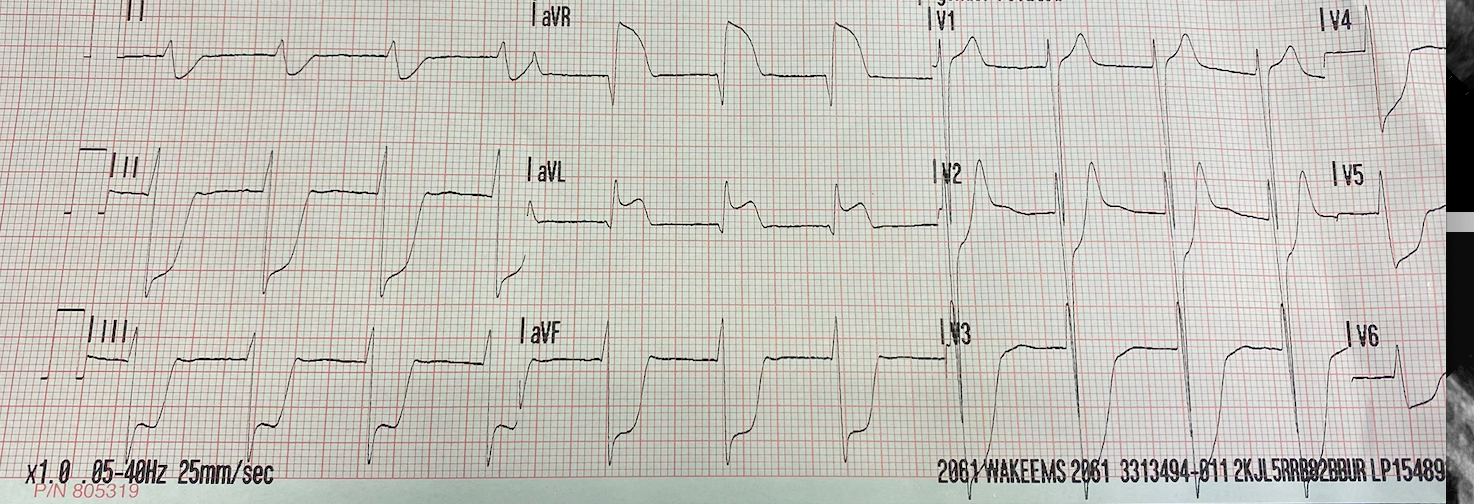
Figure 1: Initial EKG tracing by Emergency Medical Services showing significant ST elevations in aVR and aVL with diffuse ST depression.
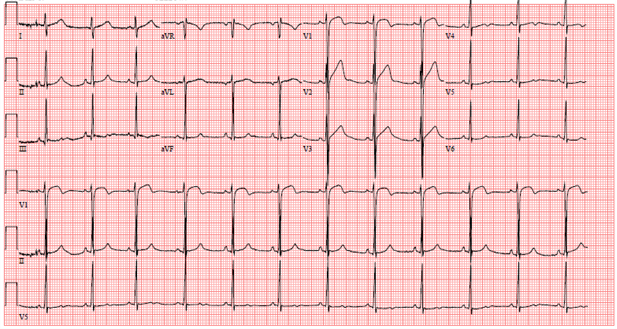
Figure 2: EKG on arrival to outside hospital Emergency Department showing residual ST elevation in V1, hyperacute T waves in V2-V3, with ST segment flattening in V4-V6.
A posteroanterior chest X-ray (Figure 3) and transthoracic echocardiogram were both obtained without acute findings. Transthoracic echocardiography was notable for normal biventricular size, wall thickness, and systolic function (Movie 1). Coronaries were also noted to have normal origin and course.
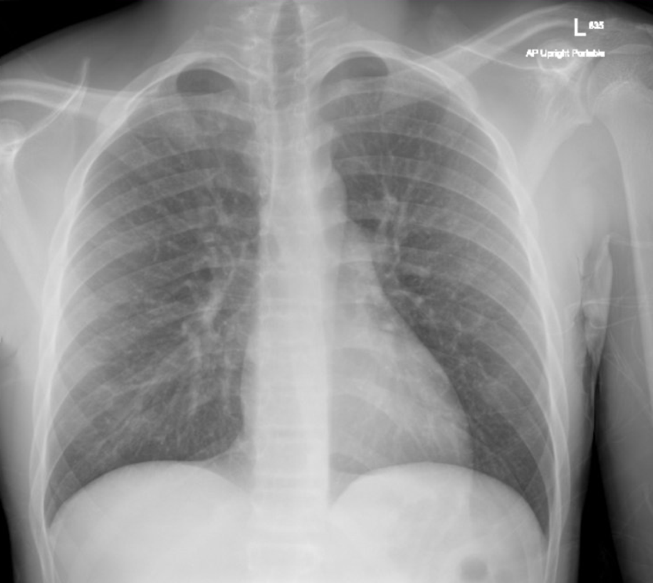
Figure 3: Chest x-ray without acute cardiopulmonary findings.
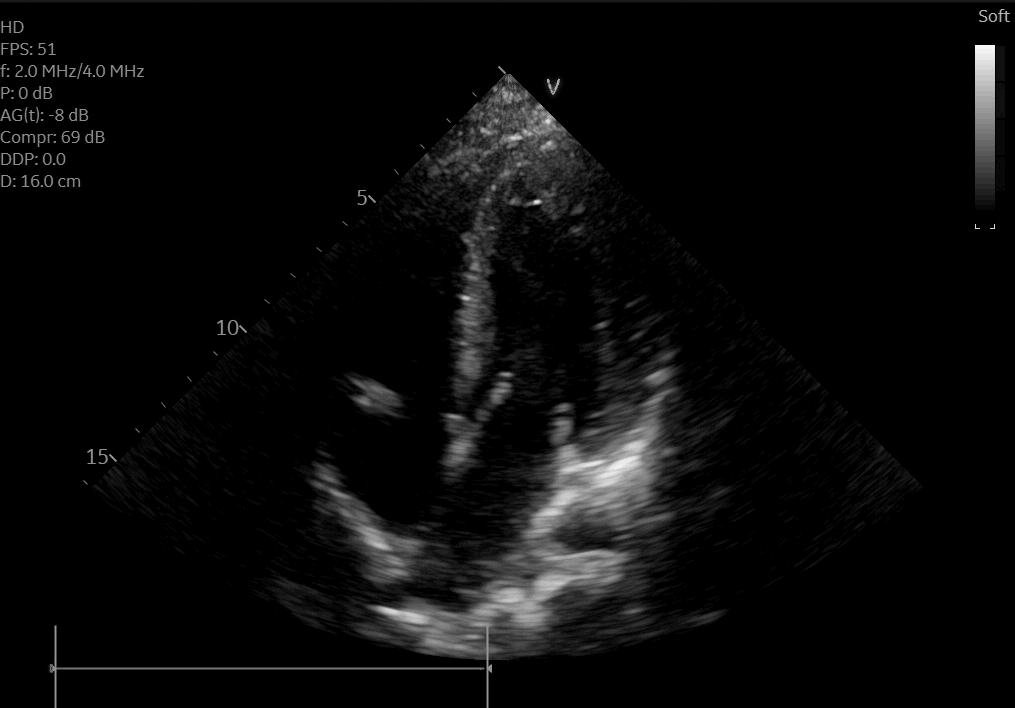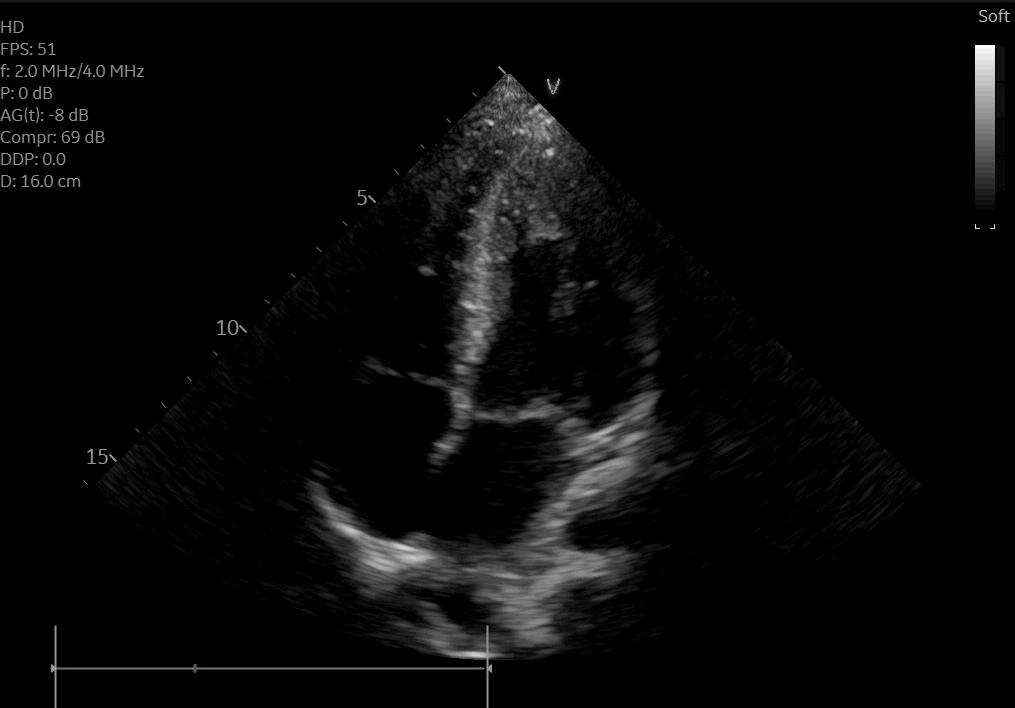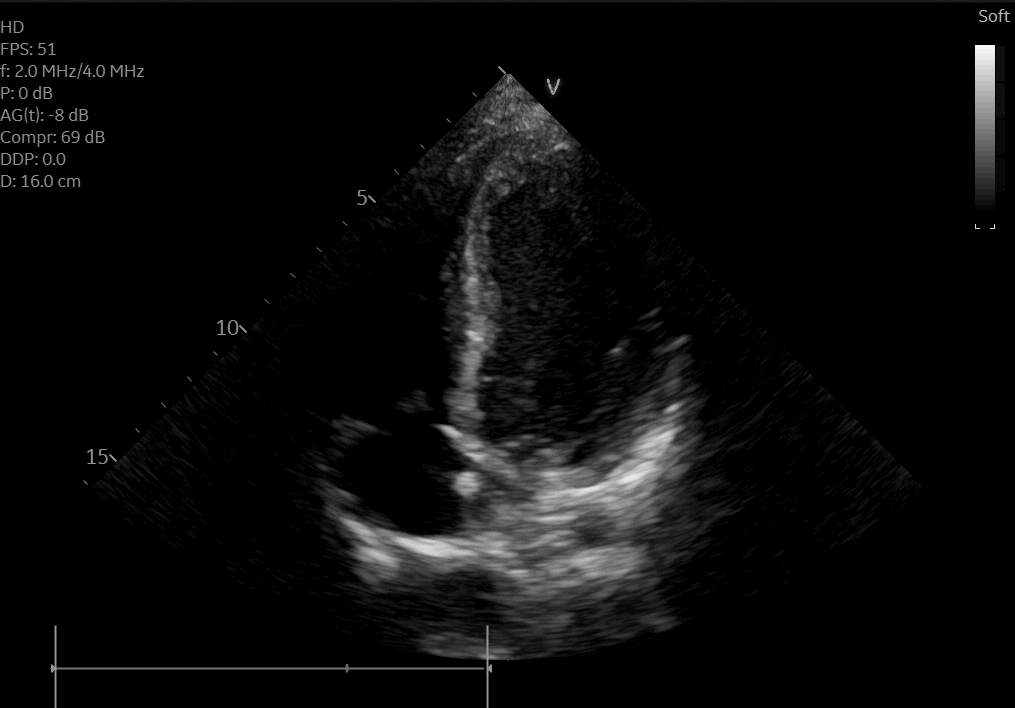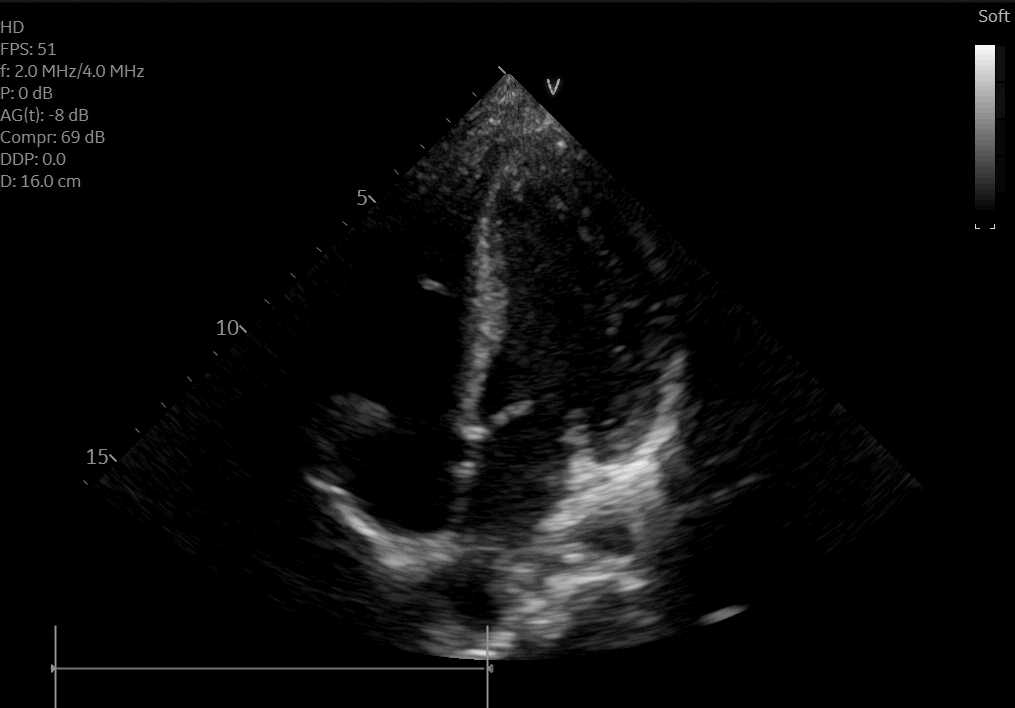
Movie 1: Echocardiogram apical four chamber view showing preserved biventricular function.
Because of reportedly normal prior echocardiography, a cardiac MRI (CMR) was obtained for recurrent, exertional syncope.
CMR Findings (1.5T):
Cine imaging demonstrated normal LV cavity size and hyperdynamic systolic function. Quantitative left ventricular EF was calculated at 78% and right ventricular EF was also normal (Movie 2). There were no regional wall motion abnormalities. The right ventricle was normal in cavity size, wall thickness, and systolic function.
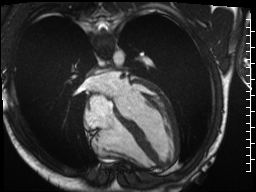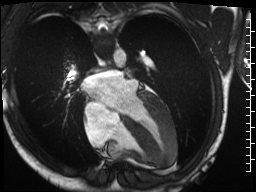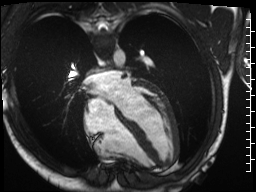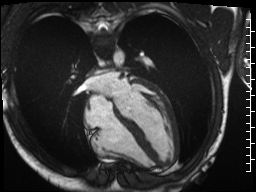
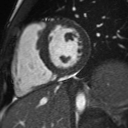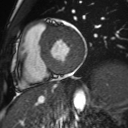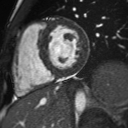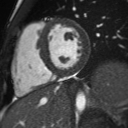
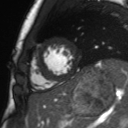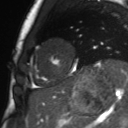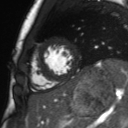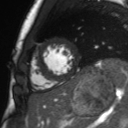
Movie 2: Long and short axis cine SSFP images show preserved biventricular function. LV volumes and segmental wall motion are normal.
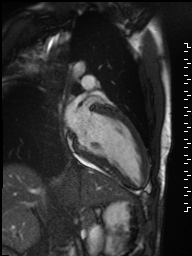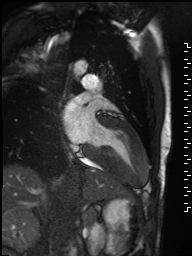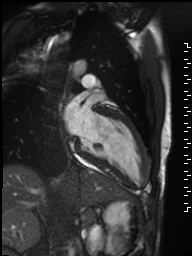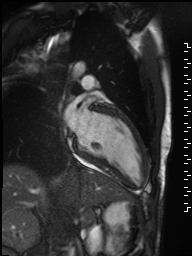
Localized hypertrophy was noted involving only the basal inferior septum, which measured 1.3 cm in maximum wall thickness (Figure 4). There were no other findings to suggest hypertrophic cardiomyopathy.
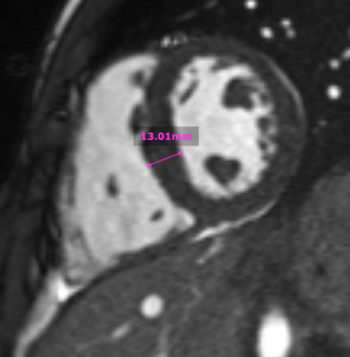
Figure 4: Short axis cine SSFP image with mild hypertrophy of the basal inferior septum (1.3cm), but no other areas of hypertrophy.
Native T1 maps did not reveal an elevation in T1 (Figure 5). Likewise, T2 mapping did not reveal an elevation in T2 (Figure 6).
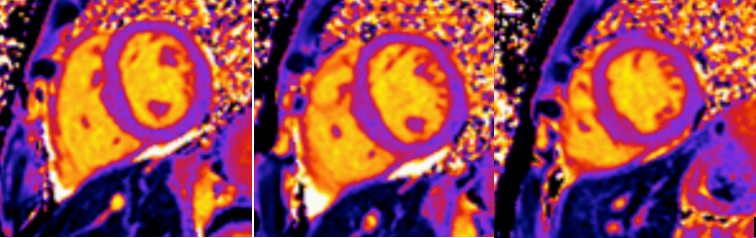
Figure 5: Mid-ventricular short axis T1 maps did not demonstrate any abnormalities.
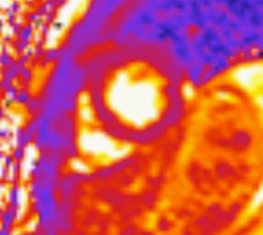
Figure 6: Mid-ventricular short axis T2 maps did not demonstrate any abnormalities.
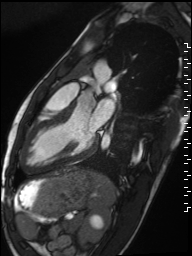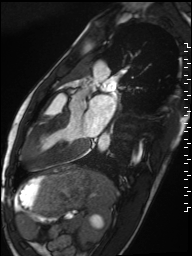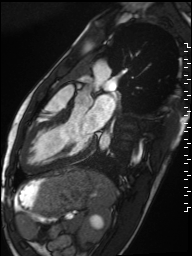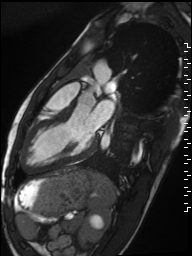
Following the administration of gadolinium contrast, single shot delayed enhancement imaging was performed using a long inversion time (TI = 600 ms, 1.5T) to screen for intracardiac thrombus. No intracardiac thrombus was visualized (Figure 7).
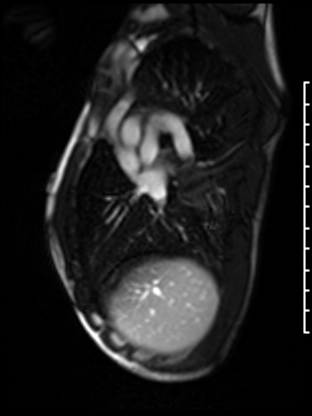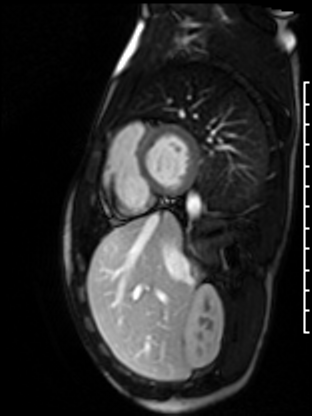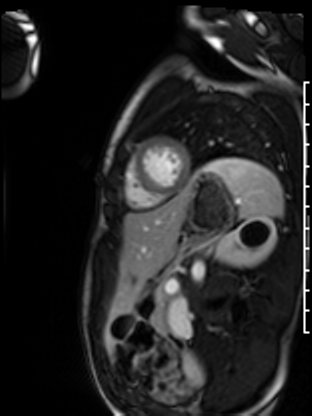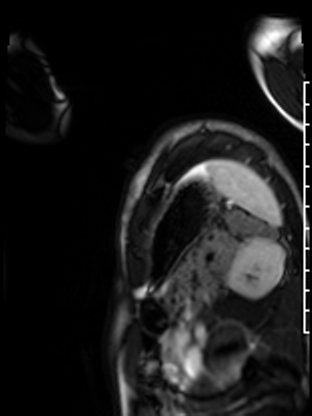
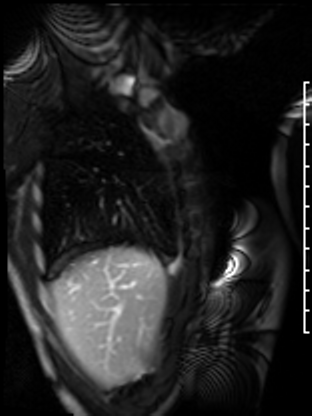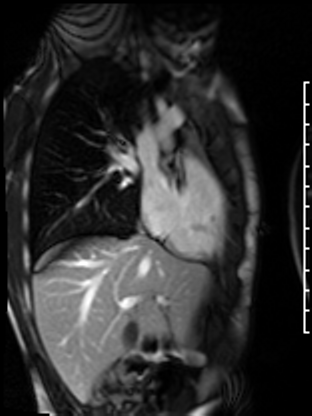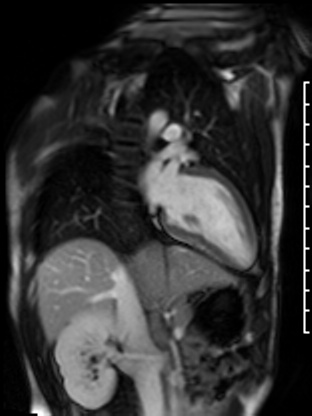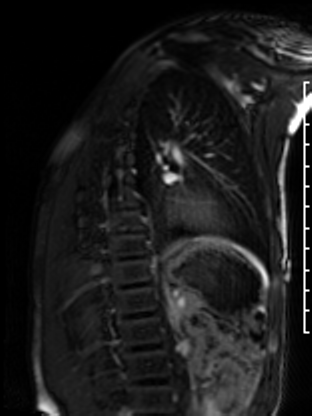
Figure 7: Long and short axis cine SSFP images following gadolinium contrast with long inversion time reveal no intracardiac thrombus.
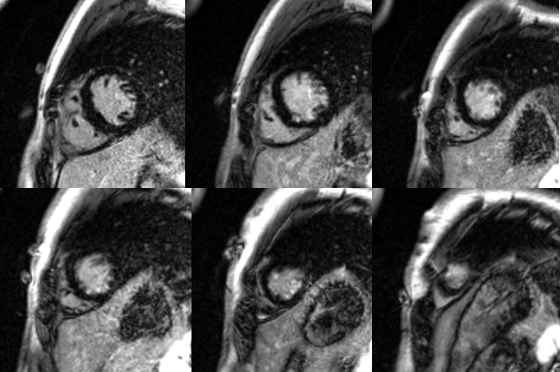
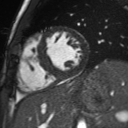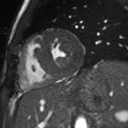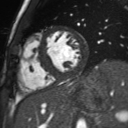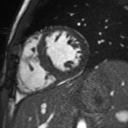
Figure 8: Basal to apical short axis delayed enhancement images demonstrate subendocardial hyperenhancement in the mid and apical LV inferior wall.
Flow independent dark blood delayed enhancement (FIDDLE) imaging sequences were then obtained. This confirmed that there was focal, subendocardial hyperenhancement involving the LV mid-apical inferoseptum and inferior walls, and the LV apex. This pattern was most concerning for myocardial infarction in the LAD perfusion territory (Figure 9, arrows).
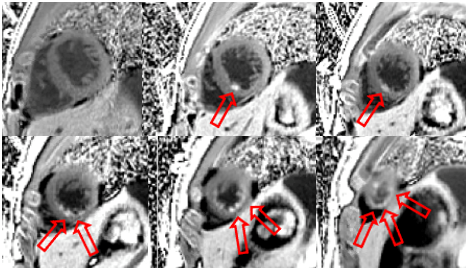
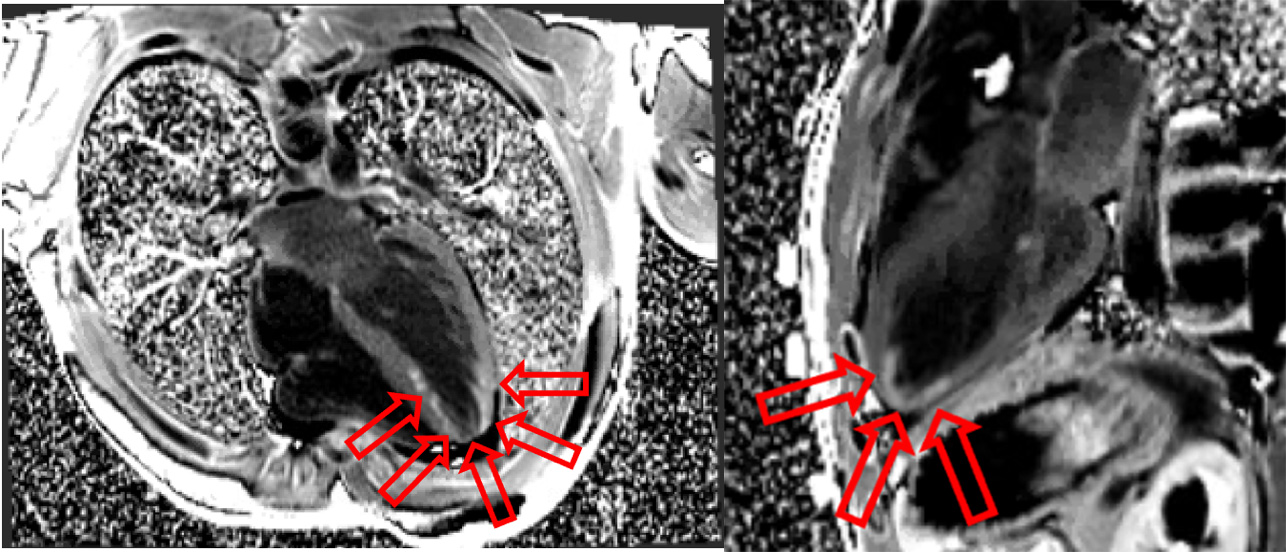
Figure 9: Flow independent dark blood delayed enhancement (FIDDLE) images characterize the punctate subendocardial enhancement in the LV mid-apical inferoseptum and inferior walls, as well as in the LV apex (arrows).
Conclusion:
This patient’s CMR findings of hyperenhancement in the LAD territory in the setting of unexplained syncope are most concerning for a coronary event. The differential includes acute coronary syndrome due to embolic or thrombotic event, coronary vasospasm, and/or coronary dissection. Unfortunately, the origin of the left main coronary artery was not well seen by CMR/MRA. A coronary angiogram was subsequently performed the following day (Figure 10) in addition to CT coronary angiography ( Figure 11).
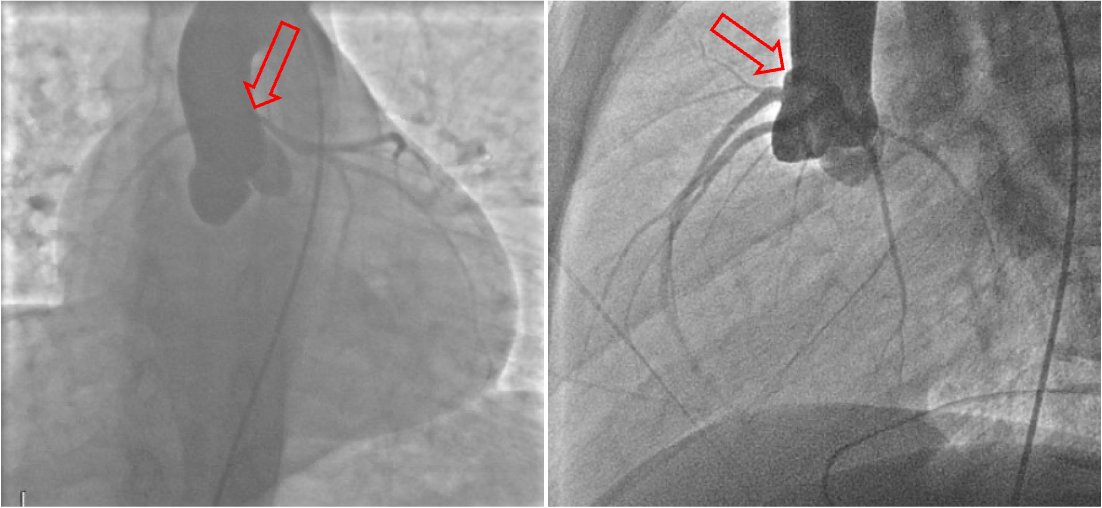
Figure 10: Coronary angiogram shows normal origin of the right coronary artery and concern for anomalous origin of the left main coronary artery (arrows). Otherwise, no obstructive coronary disease.
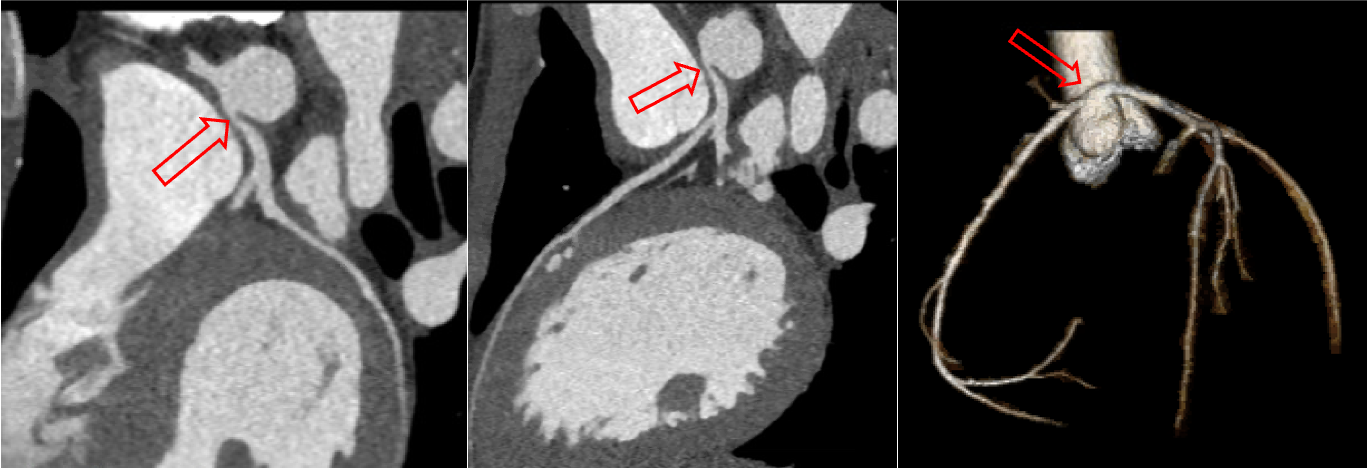
Figure 11: CT angiogram demonstrating anomalous left coronary artery arising from the right coronary sinus of Valsalva (arrows) with high-risk features including slit-like orifice, inter-arterial course, and likely intramural segment.
These studies confirmed anomalous left coronary artery (Figure 10 and 11, arrows). By CT angiography the left coronary ostium was found to originate anteriorly off the right coronary cusp, separately from the right coronary ostia, with a slit-like orifice and inter-arterial course between the aorta and main pulmonary artery. The proximal portion of the left main coronary artery was also noted to have a 3 mm narrowed segment with lack of adjacent coronary fat, likely reflecting a short intramural segment. These morphological features including inter-arterial course, intramural course, and slit-like ostium have all been reported in the literature as high-risk CT findings for sudden cardiac death in children with anomalous aortic origin of a coronary artery.
The patient was ultimately referred to cardiac surgery and underwent successful unroofing of the left coronary artery during his index hospitalization. He has done well postoperatively and was discharged home on post-operative day three. His echocardiogram remained stable with normal biventricular function and patency of the left coronary ostium. He has thus far remained symptom free with plans for repeat cardiac MRI stress testing in 3 months to determine eligibility for participation in sports.
Perspective:
Congenital anomalies of the coronary arteries are common, occurring in approximately 0.3% – 1.3% of the population [1]. While most cases are clinically insignificant, some anomalies have been associated with increased risk of sudden cardiac death (SCD), especially during or following a period of strenuous exercise. The presumptive mechanism of SCD in these patients is myocardial ischemia. This discussion will highlight the high-risk features of anomalous coronary arteries as well as the cardiac MRI findings which may aid in diagnosis of these patients.
The highest-risk coronary anomalies are believed to be those in which the anomalous coronary artery arises from the opposite aortic sinus and courses inter-arterially between the great vessels [2], as seen in this case. The mechanism of ischemia in these patients is still ambiguous, but thought to be due to a slit-like ostium, acute take-off angle, proximal hypoplasia, and/or lateral compression of an intramural segment of the anomalous vessel [3]. Some patients may have ischemic symptoms, including syncope and angina, although up to 66% will be asymptomatic with initial presentation being SCD [4].
In autopsy studies, young athletes with SCD due to coronary anomalies were found to have pathologic evidence of both acute and chronic myocardial ischemic injury with resulting myocardial fibrosis and scar formation [4]. This is likely the result of repetitive ischemic events over the patient’s lifetime. In this setting, cardiac MRI and late gadolinium enhancement imaging (LGE) techniques may aid in diagnosis of congenital anomalies by detection of myocardial scar.
Cases of patients with hyperenhancement on LGE imaging due to congenital coronary anomalies have been reported [5,6]. In these cases, hyperenhancement has been focal, subendocardial, and within the distribution of the patient’s anomalous coronary artery. This is consistent with a typical pattern of myocardial ischemia and similar to the imaging findings in our case. Still, more data will be needed to determine the impact of this myocardial scarring/fibrosis for these patient’s long term.
Additionally, t his pattern of LGE varies from other common causes of hyperenhancement in young adults which may present with similar symptoms, including hypertrophic cardiomyopathy and myocarditis. In myocarditis, LGE hyperenhancement is typically patchy, sub-epicardial, and characteristically does not follow a coronary distribution [7]. Alternatively, the most common location for hyperenhancement in hypertrophic cardiomyopathy is within the septum or RV insertion points [8]. Hyperenhancement is also localized to areas of hypertrophy in the majority of HCM patients [8].
Available studies suggest that surgical repair offers a safe and effective strategy to relieve ischemic symptoms for patients with congenital coronary anomalies, although long-term data on reduction of SCD risk has not yet been established [9]. Current consensus guidelines recommend surgical revascularization for patients with an anomalous left coronary artery from the right aortic cusp regardless of ischemia or symptoms [9]. Surgical revascularization for patients with an anomalous right coronary artery from the left aortic cusp is indicated in the setting of symptoms concerning for ischemia, documented ischemia, and ventricular arrhythmias. Surgical intervention for the asymptomatic patient with this finding is more controversial [9].
The decision for patients to return to competitive sports after surgical correction has also been addressed in recent guidelines. Specifically, a 2015 scientific statement by the American Heart Association/American College of Cardiology recommends a waiting period of 3 months post successful surgical correction for children with anomalous origin from the wrong sinus prior to considering return to competitive sports. Additionally, the patient should continue to be clinically symptom free with negative exercise stress testing prior to participation [10]. Similarly, the European Association of Preventive Cardiology 2019 guidelines recommend risk stratification of all adult athletes with a history of coronary artery disease or myocardial infarction as in our case. Low risk adults that may be advised to return to sports include those with: absence of critical coronary stenosis (<70% of major coronary arteries or < 50% of left main coronary artery), LV ejection fraction > 50%, normal age-adjusted exercise capacity, and negative exercise stress testing for inducible ischemia or ventricular arrythmia [11].
Anomalous coronary artery detection remains challenging and demands a high clinical index of suspicion and low threshold for acquisition of multi-modality imaging in cases of unexplained exertional symptoms, primarily syncope and angina, in young adults. This case highlights the diagnostic utility of LGE imaging in patients with congenital coronary anomalies. In our case, the diagnosis for this patient was made in part by late gadolinium enhancement on cardiac MRI using FIDDLE [12].
Click here to view the images on CloudCMR
References:
- Click RL, Holmes DR Jr, Vlietstra RE, Kosinski AS, Kronmal RA. Anomalous coronary arteries: location, degree of atherosclerosis and effect on survival–a report from the Coronary Artery Surgery Study. J Am Coll Cardiol . 1989;13(3):531-537. doi:10.1016/0735-1097(89)90588-3
- Mirchandani S, Phoon CK. Management of anomalous coronary arteries from the contralateral sinus. Int J Cardiol . 2005;102(3):383-389. doi:10.1016/j.ijcard.2004.10.010.
- Bigler MR, Ashraf A, Seiler C, et al. Hemodynamic Relevance of Anomalous Coronary Arteries Originating From the Opposite Sinus of Valsalva-In Search of the Evidence. Front Cardiovasc Med . 2021;7:591326. Published 2021 Jan 21. doi:10.3389/fcvm.2020.591326.
- Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol . 2000;35(6):1493-1501. doi:10.1016/s0735-1097(00)00566-0.
- Suekuni H, Kido T, Shiraishi Y, et al. Detecting a subendocardial infarction in a child with coronary anomaly by three-dimensional late gadolinium enhancement MRI using compressed sensing. Radiol Case Rep . 2020;16(2):377-380. Published 2020 Dec 8. doi:10.1016/j.radcr.2020.11.048.
- Nazir MS, Preston R, Chiribiri A, Sheikh N. Multimodal Imaging for Diagnosis of Anomalous Coronary Artery With Subsequent Myocardial Infarction. JACC Case Rep . 2021;3(10):1310-1314. Published 2021 Aug 19. doi:10.1016/j.jaccas.2021.06.003.
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol . 2009;53(17):1475-1487. doi:10.1016/j.jacc.2009.02.007.
- Axelsson Raja A, Farhad H, Valente AM, et al. Prevalence and Progression of Late Gadolinium Enhancement in Children and Adolescents With Hypertrophic Cardiomyopathy. Circulation . 2018;138(8):782-792. doi:10.1161/CIRCULATIONAHA.117.032966.
- Nguyen, A.L., Haas, F., Evens, J. et al. Sudden cardiac death after repair of anomalous origin of left coronary artery from right sinus of Valsalva with an interarterial course. Neth Heart J 20, 463–471 (2012). https://doi.org/10.1007/s12471-012-0324-4.
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol. 2019 May 14;73(18):2361-2362]. J Am Coll Cardiol . 2019;73(12):e81-e192. doi:10.1016/j.jacc.2018.08.1029.
- Maron, B. J., D. P. Zipes and R. J. Kovacs (2015). “Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Preamble, Principles, and General Considerations: A Scientific Statement From the American Heart Association and American College of Cardiology.” J Am Coll Cardiol 66(21):2343-2349.
- Borjesson, M., M. Dellborg, J. Niebauer, A. LaGerche, C. Schmied, E. E. Solberg, M. Halle, P. E. Adami, A. Biffi, F. Carré, S. Caselli, M. Papadakis, A. Pressler, H. Rasmusen, L. Serratosa, S. Sharma, F. van Buuren and A. Pelliccia (2020). “Brief recommendations for participation in leisure time or competitive sports in athletes-patients with coronary artery disease: Summary of a Position Statement from the Sports Cardiology Section of the European Association of Preventive Cardiology (EAPC).” Eur J Prev Cardiol 27(7): 770-776.
- Kim, H.W., et al., Flow-Independent Dark-blood DeLayed Enhancement (FIDDLE): validation of a novel black blood technique for the diagnosis of myocardial infarction. Journal of Cardiovascular Magnetic Resonance, 2016. 18(S1).
Case prepared by:
Anna Baritussio, MD, PhD
Associate Editor, Cases of SCMR
Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University Hospital Padua, Padua, Italy







