A Case of Myocardial Infarction in a Pediatric Patient with Homozygous Hypercholoesteremia after Supravalvar Stenosis Repair and Bilateral Coronary Ostial Plasty
Addison Gearhart, MD, Sarah D de Ferranti, MD MPH, Rebecca Beroukhim, MD
Department of Cardiology, Boston Children’s Hospital, Boston, MA, USA
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
Clinical History:
An 11-year-old female with homozygous dominant familial hypercholesterolemia (HoFH) secondary to a LDLR receptor mutation, associated supravalvar stenosis with increasing left ventricular outflow tract obstruction (LVOTO), and rare episodes of exertional chest pain and dizziness was referred for surgical evaluation. An echocardiogram revealed a mildly hypoplastic tricommissural aortic valve, moderately hypoplastic sinotubular junction, an echogenic lucency along the ascending aorta wall, mild aortic regurgitation (AR) and moderate to severe LVOTO, mildly increased left ventricular (LV) septal thickness with normal LV mass, size, and systolic function with an absence of regional wall motion abnormalities (Figure 1). Her total cholesterol on presentation was >800 mg/dL (reference: 170 mg/dL). A routine preoperative coronary angiogram showed 50% stenosis of the right coronary artery (RCA) ostium and normal left main coronary artery (LMCA). Her cardiac CT re-demonstrated a mildly hypoplastic aortic valve annulus, moderate narrowing of the supravalvar area with mild post stenotic dilation of the ascending aorta, a non-circumferential patchy calcification in the supravalvar area and an RCA with relatively focal, luminal narrowing just distal to its take off (Figure 2).
She underwent a planned 3-patch supravalvar plasty, ascending aortoplasty with plaque resection, and removal of non-coronary sinus calcification. Intraoperative inspection revealed a thick and fibrotic calcified aortic wall that extended anteriorly toward the LMCA and RCA ostia, infiltrating both. As a result, she underwent RCA and LCA ostial plasty. On the first post-operative day, she developed shortness of breath, low cardiac output, chest pain, new onset dynamic submillimeter inferior ST segment elevation, and an elevated high sensitivity troponin with a peak of 8.28 ng/mL (critical high >0.09 ng/mL); Figure 3. An emergent echocardiogram demonstrated new dyskinesia of the basal aspect of the inferior septum concerning for a myocardial infarction of the RCA. Though coronary angiography showed widely patent bilateral coronary ostia, there was approximately 50% stenosis at the distal end of the RCA patch (~5 mm from the ostia) where a small protrusion extended into the vessel, raising concern for a mid-proximal fibrous plaque dislodgement. The distal RCA vasculature and immediate ostia of the LMCA were unobstructed (TIMI flow grade 3). The distal LMCA narrowed to as much as 40%, just proximal to the bifurcation of the circumflex (LCX), with unobstructed left anterior descending (LAD) and distal vasculature. Due to the fresh surgical patch and concerns for plaque calcification and ongoing potential ischemia, the decision was made to return to the operating room. Intraoperative findings suggested that the mid-proximal RCA narrowing was likely due to an intimal fold in the RCA from a trivial residual edge of the native coronary, less than 0.5 mm in length, that protruded into the RCA orifice. She was medically stabilized with heparin and vasoactive infusions and underwent emergent revision and extension of the RCA ostial plasty site and reduction of the non-coronary sinus to decrease the degree of AR.
Over the next two years, she developed progressive mitral regurgitation (MR), AR, and LV dilation. Serial surveillance positron emission tomography (PET) scans to evaluate for inducible ischemia showed a medium sized fixed perfusion defect of severe intensity in the basal inferior and inferoseptal LV myocardium consistent with a prior MI of the RCA territory (Figure 4). There was no evidence of residual stress induced ischemia. Regional and global stress myocardial blood flow and flow reserve for the RCA were reduced for her age, consistent with an infarct. Of note, the LCX and global LV stress myocardial blood flow were also reduced, consistent increased wall stress.

Figure 1: Transthoracic echocardiogram. 1A. Apical 3-chamber view showing moderate-to-severe multilevel left ventricular outflow tract obstruction with a mildly hypoplastic aortic valve and moderately hypoplastic sino-tubular junction. 1B: Parasternal long axis view demonstrating the aortic valve leaflets are thickened and doming, the sino-tubular junction is moderately hypoplastic, and a linear echodensity is noted along the wall of the ascending aorta consistent with atherosclerosis. There is mild aortic regurgitation with a vena contracta measuring 2.8 mm.
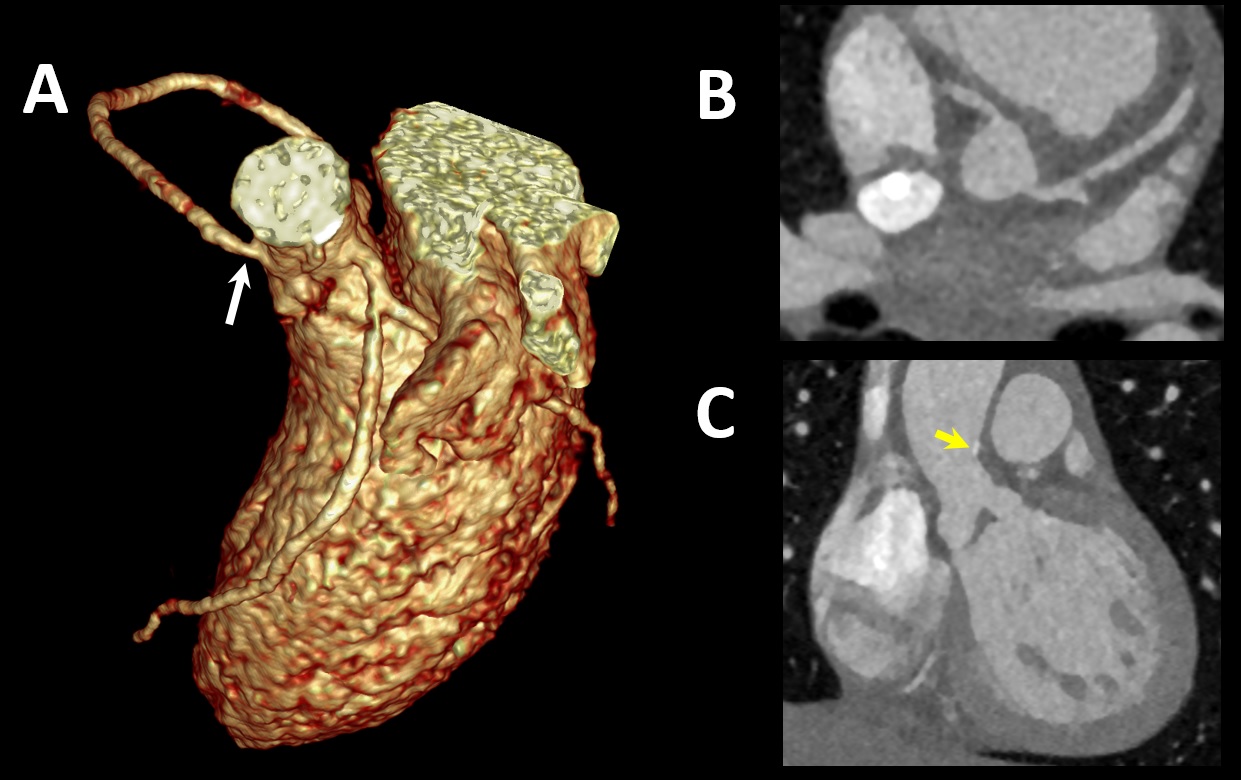
Figure 2: Preoperative Cardiac CT. 3D reconstruction (A) and aortic valve en face view (B). The RCA has a relatively focal, luminal narrowing just distal to its take off. Off-axis coronal view (C). The aortic valve leaflets are thick and abnormal, with calcified plaque in the proximal ascending aorta (yellow arrow).
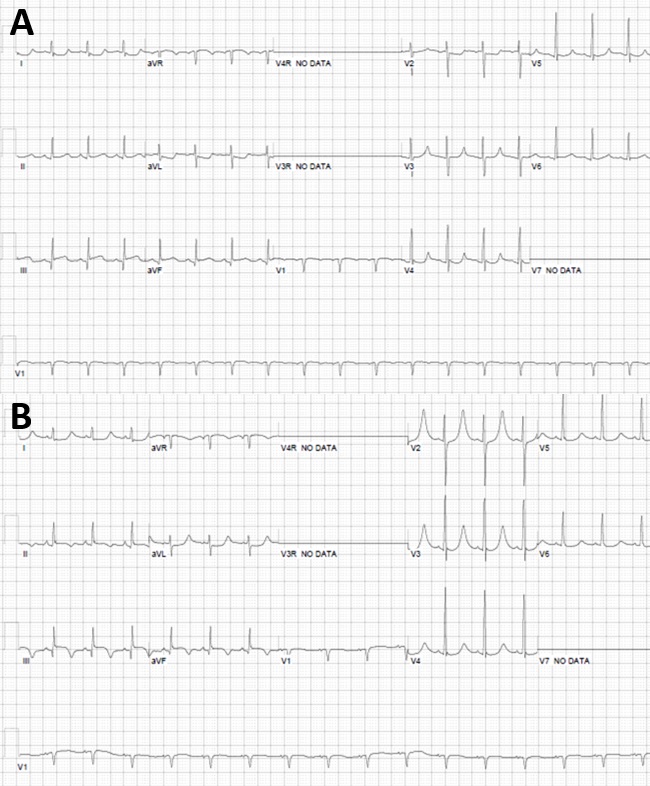
Figure 3: Serial 12 lead electrocardiograms (A,B). Submillimeter inferior ST elevation in lead III with q wave and reciprocal ST segment depressions laterally (A) and hyperacute T waves in V2 and V3 (B) consistent evolution of inferior transmural MI.

Figure 4: Cardiac positron emission tomography study. Medium sized fixed perfusion defect of severe intensity in the basal inferior and inferoseptal walls (arrows) consistent with a prior MI in the RCA territory. There is no evidence of residual stress induced ischemia. Additionally the study concluded that both regional and global stress myocardial blood flow and flow reserve (MBF) for the RCA were reduced for her age consistent with a prior RCA infarct.
CMR Findings
Serial CMR (1.5 T, Ingenia, Philips Healthcare, Best, Netherlands) studies over the course of two years following her initial repair showed progressive mild MR (regurgitant fraction 25%; up from 22% year prior), moderate AR (regurgitant fraction of 34% up from 28% year prior), moderately-to-severely dilated left ventricle (LVEDVi 148.8ml/m2; z-score 6.6, up from 130.5ml/m2; z-score 4.9 the year prior) with borderline depressed systolic function of 52%. There were stable regional wall motion abnormalities (RWMA) of the basal and mid-cavity inferoseptal and inferior wall segments and papillary muscles (AHA segments 3-6, 9 and 10) with associated thinning and hypokinesis. (Movie 1). These regions corresponded with areas of mostly subendocardial late gadolinium enhancement (LGE) with transmural enhancement (75%-100% wall thickness) in the basal segments, consistent with a prior RCA infarct (Figure 5). Left ventricular LGE mass was 30.2 grams, 27%. The right ventricular global systolic function was normal.

Movie 1. Cine steady state free precession 4 chamber view. Regional wall motion abnormalities of the basal and mid-cavity inferoseptal wall segments with associated thinning and hypokinesis.
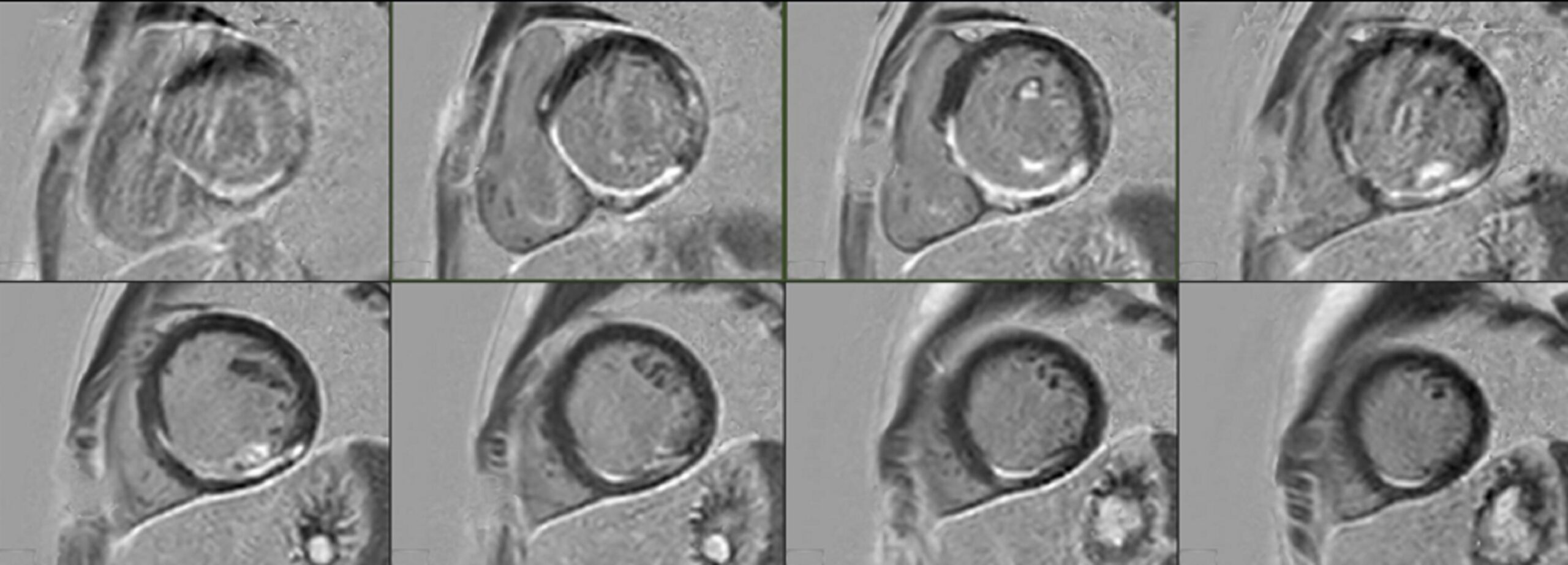
Figure 5: Cardiovascular magnetic resonance myocardial delayed enhancement short axis stack. Subendocardial late gadolinium enhancement in the inferoseptal and inferior walls of the left ventricle, in a distal right coronary artery distribution.
Conclusions:
Homozygous dominant familial hypercholesterolemia is a rare and underdiagnosed autosomal dominant genetic disease associated with pediatric onset aortic valvar and supravalvar disease and multivessel coronary artery disease. Coronary plaques are progressive, prone to rupture, and may become dislodged during surgical intervention. In the presence of clinical decompensation, new EKG findings, and RWMAs, it is imperative to rule out an obstructive coronary process. Multimodality imaging provides a comprehensive evaluation for the acute diagnosis of coronary obstruction and lifelong surveillance. CMR maintains a particularly important role for the clinical management of pediatric patients after an MI who have co-existing congenital heart disease.
Following serial CMRs showing progressive AR, MR, and a mild decline in LV function, at 13 years of age, the patient ultimately underwent a Ross procedure, partial Konno, reimplantation of coronaries to pulmonary autograft, RV-PA conduit placement and mitral valvuloplasty with 28 mm Sorin ring. At her most recent clinical visit, she denies cardiac symptoms. The ongoing medical management of three co-existing disease processes (e.g. coronary artery disease (CAD), AR, and MR) in a pediatric patient is not well defined, and is determined on an individual level. Given her young age and robust secondary prevention measures undertaken (high potency statin, ezetimibe, apheresis and potential future candidacy of a PCSK9 inhibitor), along with prudent medical management of ischemic heart disease, it is hopeful that her LV function will remain stable.
Perspective:
We present a pediatric case of HoFH status post supravalvar AS, aortic valve, RCA and LMCA patch repairs who suffered a non-ST segment elevation MI on postoperative day 1. Homozygous familial hypercholesterolemia is a rare and life-threatening disease characterized clinically by plasma cholesterol levels >13 mmol/L (>500 mg/dL), extensive xanthomas, and marked premature and progressive atherosclerotic cardiovascular disease (ACVD) due to gene mutations affecting LDL metabolism.(1) Our patient harbored an autosomal dominant LDL receptor gene discovered at 6 years of age during a screening for a strong family history of paternal and maternal relatives with hypercholesterolemia, premature MI, and coronary artery bypass grafts.
Premature malignant atherogenesis can lead to pediatric onset aortic root abnormalities and multivessel coronary heart disease. Untreated, most patients with significantly elevated LDL cholesterol levels acquire overt atherosclerosis before the age of 10 years, and many much earlier – patients may not survive past 30 years.(2) Our patient had a total cholesterol of >800mg/dL on presentation and was actively being treated with the recommended LDL apheresis, statins and cholesterol absorption inhibitors. The primary goals of management are prevention of ACVD by prompt and comprehensive control of hypercholesterolemia, and early recognition of complications, particularly ostial occlusion and supravalvar and valvar aortic stenosis.(3) Unfortunately, HoFH is often diagnosed late, when substantial coronary atherosclerosis has already ensued, underscoring the benefits of treatment optimization in childhood and access to experienced pediatric centers.
Acute postoperative transmural infarct of the RCA territory in the setting of known underlying aggressive atherosclerotic disease is a rarely encountered clinical scenario in pediatrics and elicited significant discussion on the optimal management strategy. Given angiographic and EKG findings in the setting of hypokinetic and ischemic myocardium, significant concern was raised for destabilization of the known atherosclerotic lesion at the margin of the surgical patch. Management options discussed included high-risk percutaneous coronary intervention (PCI) with coronary stent, aggressive medical management or operative re-exploration and re-operation coronary plasty. In the setting of a fresh suture line and potential of thrombus formation, PCI was deemed too high risk. Furthermore, adequate stent apposition would be paramount in a patient with HoFH already at heightened risk for restenosis and recurrent disease. Intensive medical management of non-ST segment elevation myocardial infarction (NSTEMI) with 72 hours of systemic anticoagulation and 3-6 months of dual anti-platelet therapy was also considered; however, extrapolation from PCI data favored improved morbidity and mortality with early invasive revascularization in the setting of NSTEMI.(4)
Management of this case was complicated by the coexistence of supravalvar stenosis and progressive AR and MR, in addition to ischemic cardiomyopathy. Although multimodality imaging was critical in the early preoperative and postoperative course, CMR was integral to her ongoing disease surveillance and decision-making to proceed with surgical aortic and mitral valve repairs. CMR has been considered the gold standard in assessment of ventricular volume and function.(5) While transthoracic echocardiography remains the imaging modality of choice for valve assessment, an increasing number of studies have favorably evaluated the reliability and accuracy of CMR in pediatric valvular heart disease using CMR phase contrast imaging.(6,7) Furthermore, the transmural extent of LGE on CMR is a well-established method to predict the reversibility of myocardial dysfunction.(8)
Click here to view the entire CMR on CloudCMR.
References
- Cuchel M., Bruckert E., Ginsberg HN., et al. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper fromthe Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014. Doi: 10.1093/eurheartj/ehu274.
- Wiederschain GY. The metabolic and molecular bases of inherited disease. Biochem 2002. Doi: 10.1023/A:1017418800320.
- Kolansky DM., Cuchel M., Clark BJ., et al. Longitudinal Evaluation and Assessment of Cardiovascular Disease in Patients With Homozygous Familial Hypercholesterolemia. Am J Cardiol 2008. 102(11):1438-43 Doi: 10.1016/j.amjcard.2008.07.035.
- Arora S., Matsushita K., Qamar A., Stacey RB., Caughey MC. Early versus late percutaneous revascularization in patients hospitalized with non ST-segment elevation myocardial infarction: The atherosclerosis risk in communities surveillance study. Catheter Cardiovasc Interv 2018. 91(2):253-259 Doi: 10.1002/ccd.27156.
- Fratz S., Chung T., Greil GF., et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013. 15(1):51 Doi: 10.1186/1532-429X-15-51.
- Powell AJ., Maier SE., Chung T., Geva T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: In vitro and in vivo validation. Pediatr Cardiol 2000. 21(2):104-10. Doi: 10.1007/s002469910014.
- Banka P., Geva T. Advances in pediatric cardiac MRI. Curr Opin Pediatr 2016. 28(5):575-83 Doi: 10.1097/MOP.0000000000000400.
- Kim RJ., Wu E., Rafael A., et al. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N Engl J Med 2000. 343(20):1445-53 Doi: 10.1056/nejm200011163432003.
Case prepared by:
Jason N. Johnson, MD MHS
Associate Editor, Cases of SCMR
Le Bonheur Children’s Hospital, University of Tennessee Health Science Center, St. Jude Children’s Research Hospital





