The utility of cardiac MRI in the diagnosis, risk stratification, and management of cardiac sarcoidosis
Dr. Jonathan Hudson1, Dr. Sorayya Kakhi2, Dr. Yousef Daryani3
1Kings College London British Heart Foundation Centre, School of Cardiovascular and Metabolic Medicine & Sciences, London, United Kingdom, London, United Kingdom
2 King’s College Hospital NHS Foundation Trust, London, United Kingdom
3Epsom & St. Heliers NHS University Hospitals Trust, London, United Kingdom
Clinical history
A 62-year-old male with a history of hypertension, presented to the Emergency Department with a 3-week history of fatigue, lethargy, and shortness of breath on exertion. He was hemodynamically stable, and a 12-lead ECG demonstrated complete heart block with a ventricular rate of 32 beats per minute (Figure 1).

Figure 1. 12-lead ECG demonstrating complete heart block with a narrow complex escape.
Baseline blood tests were within normal range apart from a c-reactive protein of 42 (CRP normal range <2). A chest x-ray showed prominent hilar lymphadenopathy (Figure 2) and a transthoracic echocardiogram revealed a left ventricular ejection fraction of 50% with bi-atrial dilatation.

Figure 2. Hilar lymphadenopathy on conventional chest x-ray.
A CT scan of the thorax demonstrated bilateral hilar lymphadenopathy, measuring up to 14 mm and multiple enlarged mediastinal lymph nodes measuring up to 2 cm (Figure 3). Cardiac PET showed intense uptake at the level of the interventricular septum, basal inferoseptal, anterolateral wall and RV free wall, suggestive of cardiac inflammation (Figure 4). A presumed diagnosis of sarcoidosis with lung and cardiac involvement was made and a cardiac MRI (cMR) was performed (Siemens Area 1.5 Tesla).

Figure 3. CT-Thorax demonstrating large hilar lymph nodes.
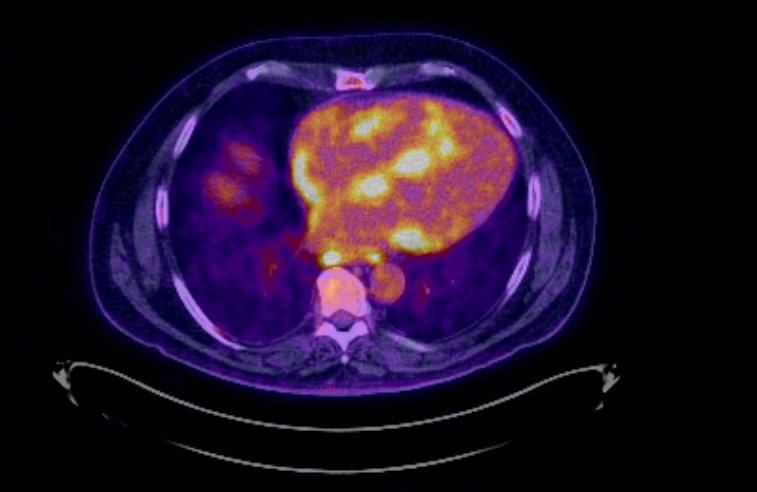
Figure 4. PET scan demonstrating intense myocardial uptake.
CMR findings
T2 weighted imaging showed increased oedema in basal antero-septum, basal infero-lateral wall, basal inferior, basal antero-lateral wall, base and mid anterior wall and septum, and likely in apex and base to mid septum (Figure 5 and 6).
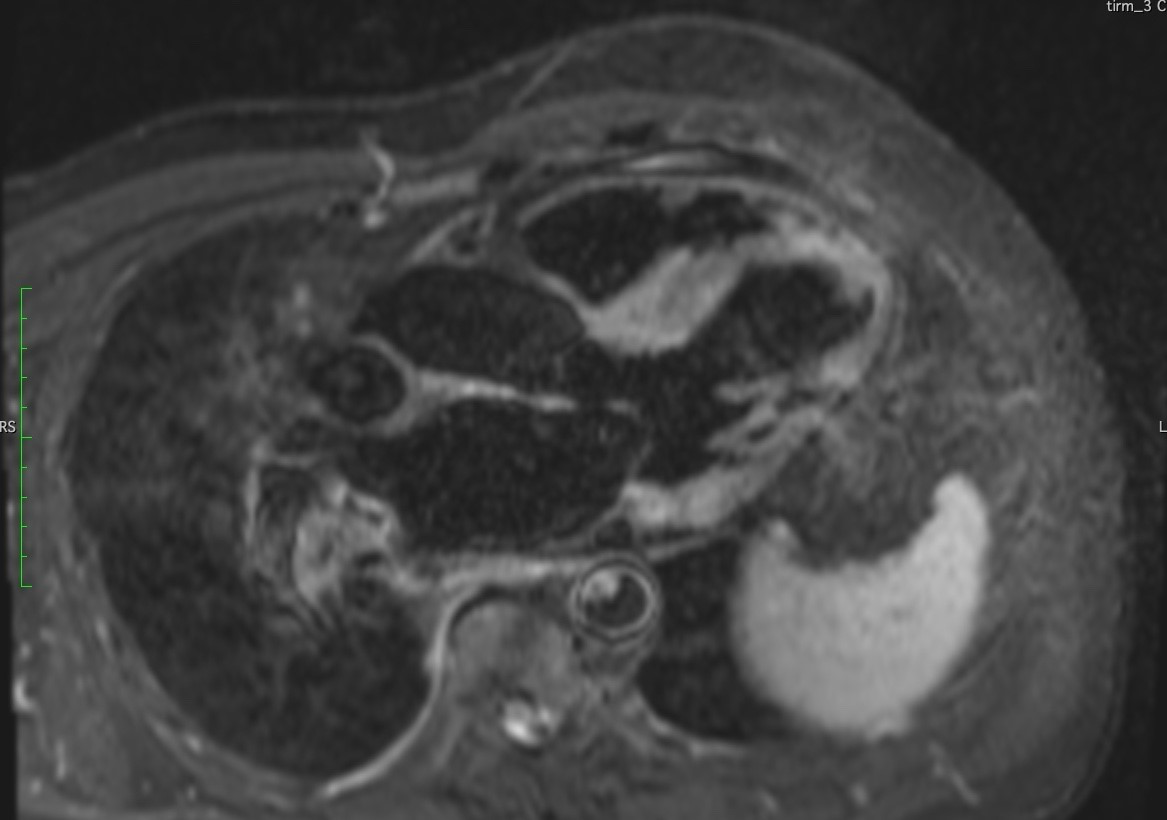
Figure 5. 3-Chamber T2 weighted image depicting edema in the antero-septal and infero-lateral wall.
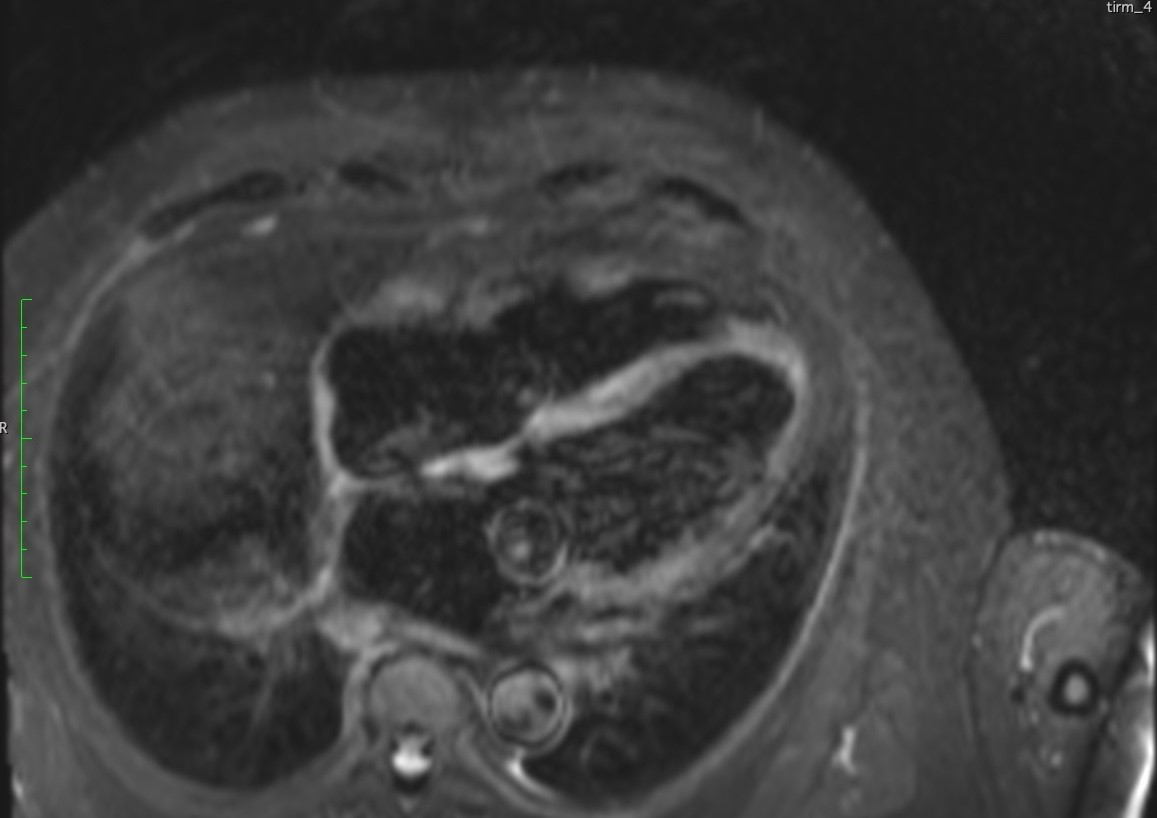
Figure 6. 4-Chamber T2 weighted image depicting edema in the septal wall.
Sub-epicardial late gadolinium enhancement (LGE) was observed in the basal anterior wall, base and mid inferior and inferolateral walls as well as the base to mid anterolateral wall, and mid wall of the base to mid septum correlating well with the LGE pattern detected. There was also uptake in the mid RV free wall, basal RV wall and left atrium. There was a mild increase in LV volume indices (LVEDV = 127mL/m2), base to mid septal hypertrophy with maximum thickness of 14 mm, and preserved LV systolic function with calculated LV ejection fraction of 56%. There was evidence of mildly reduced RV radial function with calculated RV systolic function of 43% (Figures 7-10). Moderate central mitral regurgitation was seen and corroborated with the TTE findings. The left and right atria were dilated at 77ml/m2 and 49ml/m2 respectively. Numerous lymph nodes were seen in the hilum and mid to upper mediastinum.

Figure 7. 4-Chamber LGE image depicting midway enhancement in the septal and lateral wall.
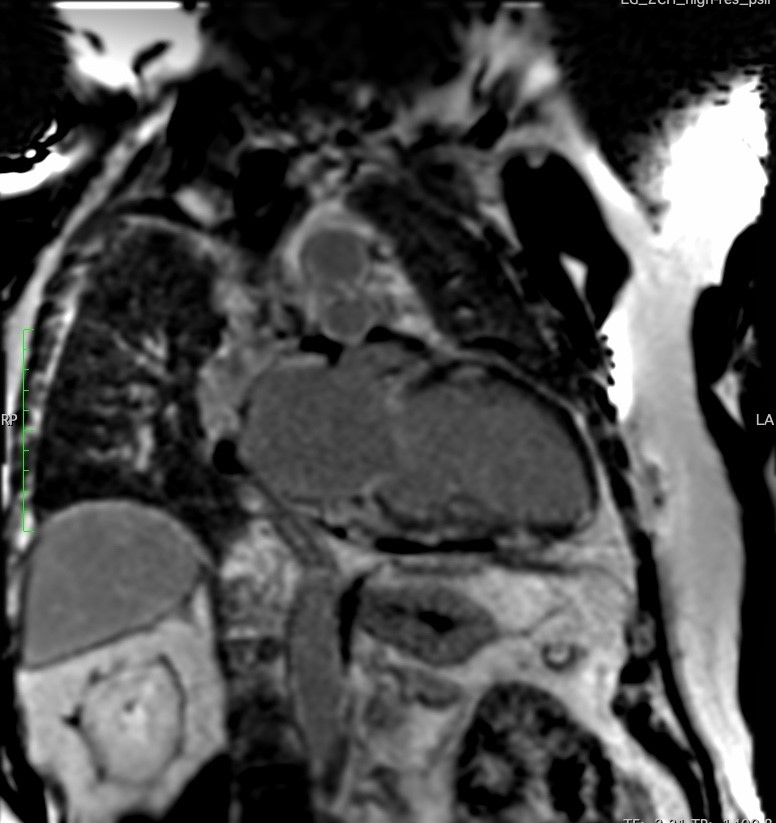
Figure 8. 2-Chamber LGE image depicting midway enhancement in the anterior wall and epicardial enhancement in the inferior wall.

Figure 9. Short-axis LGE image depicting extensive epicardial enhancement in the anterior and inferior wall.

Figure 10. Short-axis LGE image depicting midway enhancement in the LV septal wall and basal wall of the RV.
Conclusion
The patient was diagnosed with cardiac sarcoidosis complicated by complete heart block. A dual chamber ICD was implanted to treat both the high-grade AV block and prevent future fatal tachyarrhythmias. Following an uncomplicated procedure, the patient was discharged and referred for a specialist sarcoidosis centre for ongoing care. An outpatient endoscopic guided lymph node biopsy demonstrated non-caseating granulomatous tissue, confirming the diagnosis of sarcoidosis.
Perspective
This case illustrates important points regarding the investigation of patients with complete AV block and the role of cardiac MRI in making treatment decisions in cardiac sarcoidosis (CS). The patient presented in his sixth decade with a first presentation of complete AV block. The most common aetiology for this arrhythmia is progressive degeneration of the cardiac conduction system[1], however in younger patients, cardiac diseases that may be reversible or treatable should be considered such as ischaemic heart disease, cardiomyopathies, myocarditis, Lyme’s disease, congenital heart disease, electrolyte disturbances, and medications and other iatrogenic causes.[2] In patients below the age of 60 that present with unexplained high grade AV block, the possibility of CS should be considered given that as many as 34% may have a final diagnosis of CS.[3]
Symptomatic cardiac involvement is seen in 5 to 10% of patients with systematic sarcoidosis.[4] Arrhythmias including AV block, ventricular arrhythmias, and sudden cardiac death are all recognised as manifestations of CS. Sarcoidosis itself is a significant risk factor for AV block[5], which is the most common arrhythmic presentation of CS representing almost half all first symptomatic presentations.[6,7] Fatal ventricular arrhythmias are the first presentation in 15% of patients.[7]
Cardiac MRI (CMR), and in particular, late gadolinium enhancement (LGE), has become a key component of the diagnosis of CS4. LGE signifies the detection of macroscopic interstitial fibrosis that can be seen in both the acute and chronic phase of CS.[8] The pattern of LGE is most commonly nodular in appearance[9] and is located frequently in the basal and lateral segments of the midwall and subepicardium and is not confined to any specific coronary perfusion territory.[10] LGE also provides prognostic information in patients with CS, as patients with LGE are at a significantly increased risk of death from any cause as well as future arrhythmic events.[11]
Treatment of CS involves sarcoid specific immunosuppressive therapies, arrhythmia management, device therapy, and heart failure therapy where appropriate.[8] Immunosuppression in CS includes high dose corticosteroids in the early phase of disease to induce remission. This treatment has been shown to improve conduction system abnormalities by almost half of patients[12,13], and in a subset of patients will restore AV conduction function.[6] Despite the potential to reverse AV conduction disease, this effect is not consistent and thus the HRS expert statement recommends device implantation alongside immunosuppression.[4]
CMR is increasingly used to help guide decision treatments in CS4. CMR can detect areas of myocardial damage in individuals that may be asymptomatic or have preserved LV function and therefore identify patients at higher of future arrhythmias. The location of scarring may also suggest a future risk of arrhythmias as demonstrated by a study of 76 patients with cMR conformed CS. In this cohort, scars in the anterior and anteroseptal walls were associated with an increased risk of AV block while scar in the basal inferoseptum were associated with ventricular arrhythmias.[14] In patients with asymptomatic CS, the presence of LGE should lead to consideration of an electrophysiological study that could guide future management.[4]
Our patient presented with complete heart block and the decision was made to implant an ICD. For patients with CS presenting with ventricular arrhythmias or heart failure with reduced ejection despite adequate therapy, the decision to implant an ICD is straightforward.[4] However, in patients with high grade AV block only, the decision regarding ICD implantation must consider the future risk of ventricular arrhythmias or sudden cardiac death (SCD). A recent study using the Finnish CS registry indicated that the 5-year incidence of SCD of VT in patients presenting with lone AVB or lone AVB and an EF of 35-50% was 24%.[15] This study, alongside others, supports the class IIa indication provided by the HRS expert statement that patients with an indication for permanent pacing may benefit from ICD implantation.[4]
Click here to view the entire CMR on CloudCMR.
References
- Zoob M, Smith KS. The Aetiology of Complete Heart-Block. Br Med J. 1963;2(5366):1149-1153. doi:10.1136/bmj.2.5366.1149
- Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace. 2022;24(1):71-164. doi:10.1093/europace/euab232
- Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25(8):875-881. doi:10.1111/jce.12401
- Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305-1323. doi:10.1016/j.hrthm.2014.03.043
- Rosenthal DG, Fang CD, Groh CA, et al. Heart Failure, Atrioventricular Block, and Ventricular Tachycardia in Sarcoidosis. J Am Heart Assoc. 2021;10(5):e017692. doi:10.1161/JAHA.120.017692
- Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624-632. doi:10.1161/CIRCULATIONAHA.114.011522
- Ekström K, Lehtonen J, Nordenswan HK, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40(37):3121-3128. doi:10.1093/eurheartj/ehz428
- Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021;107(19):1591-1599. doi:10.1136/heartjnl-2019-316442
- Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 29(1):89-103. doi:10.1148/rg.291085052
- Vita T, Okada DR, Veillet-Chowdhury M, et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ Cardiovasc Imaging. 2018;11(1):e007030. doi:10.1161/CIRCIMAGING.117.007030
- Coleman GC, Shaw PW, Balfour PC, et al. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovasc Imaging. 2017;10(4):411-420. doi:10.1016/j.jcmg.2016.05.009
- Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29(9):1034-1041. doi:10.1016/j.cjca.2013.02.004
- Fazelpour S, Sadek MM, Nery PB, et al. Corticosteroid and Immunosuppressant Therapy for Cardiac Sarcoidosis: A Systematic Review. J Am Heart Assoc. 2021;10(17):e021183. doi:10.1161/JAHA.121.021183
- Okada DR, Xie E, Assis F, et al. Regional abnormalities on cardiac magnetic resonance imaging and arrhythmic events in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2019;30(10):1967-1976. doi:10.1111/jce.14082
- Nordenswan HK, Lehtonen J, Ekström K, et al. Outcome of Cardiac Sarcoidosis Presenting With High-Grade Atrioventricular Block. Circ Arrhythm Electrophysiol. 2018;11(8):e006145. doi:10.1161/CIRCEP.117.006145
Case prepared by Pranav Bhagirath







