Shirjeel Murtaza, MBBS and Samra Haque, MBBS
Punjab Institute of Cardiology; Lahore, Pakistan
Clinical History:
A 19 year old male presented to the outpatient department of Punjab institute of Cardiology, Lahore for evaluation. Previous medical history reveals that he had intermittent cyanosis noted by his parents for the first time five years ago along with breathing difficulties. He was then taken to a local medical facility where he was diagnosed with a large pericardial effusion. The effusion was drained for diagnostic and therapeutic purposes. Fluid analysis showed lymphocytic predominance. No organism was isolated on gram and Ziehl-Neelsen staining, however tuberculin test was positive. Considering his living in an endemic region for tuberculosis, a final diagnosis of tuberculous pericardial effusion was made. He was given anti-tuberculous therapy (isoniazid, rifampin, ethambutol, pyrazinamide) for 9 months. Subsequent multiple echocardiograms over two years showed variable amounts of pericardial effusion, but never judged to be large. Also, serial echocardiography revealed moderate to severe RV systolic dysfunction with normal LV systolic function. Thus, his diagnosis was revised to include “right ventricular failure/cardiomyopathy”. He was maintained on standard heart failure therapy and symptoms were controlled with medication. He was subsequently lost to follow up until he presented to our facility.
At our institution, a chest x-ray (Figure 1) and electrocardiogram (Figure 2) were performed initially. The chest x-ray demonstrated clear lung fields and mild cardiomegaly. ECG demonstrated large P waves indicating right atrial enlargement, right axis deviation, and prolonged PR interval.

Figure 1: Chest x-ray demonstrating clear lung fields and mild cardiomegaly

Figure 2: ECG demonstrating large “Himalayan” P waves (arrows).
A repeat echocardiogram was performed. There were no features suggesting a diagnosis of Ebstein anomaly by echocardiography. The tricuspid valve annulus was normally positioned in relation to the mitral valve annulus and the leaflets were of normal morphology. However, there was severe tricuspid insufficiency due to annular dilation along with severe right atrial dilation. The right ventricle was thin-walled with relatively scarce trabeculations and poor systolic function (Video 1). There was no evidence of pulmonary hypertension detected. The left atrial size was normal. No intracardiac shunts were detected.

Video 1: Parasternal long axis echo image showing a dilated, thinned right ventricle with depressed systolic function.
The patient’s oxygen saturations were 92-95% at rest. Given the unusual appearance of the right ventricle, CMR was planned to further characterize RV function, morphology, and viability.
CMR Findings:
Standard CMR acquisition was done on Vantage Titan 3T scanner (Canon Medical Systems, Otawara, Tochigi, Japan). Standard localizers were acquired, followed by cine SSFP in two, three and four chamber projections, short axis and axial stack cines, and right ventricular outflow tract views. Modified two and four chamber projections for additional focused views of the right ventricle were obtained. T1 weighted axial stack and fat saturation images were obtained. Gadovist contrast (Bayer HealthCare, Berlin, Germany) was given at 0.09 mL/kg dose followed by post contrast imaging at 8 minutes. Following the initial study, additional CMR images were obtained two days later for further assessment of the right ventricular anatomy.
The right atrium was severely dilated. The right ventricular base was dilated along with thinning of the RV free wall. There was virtually no RV myocardium with lack of trabeculations especially in the basal region (Videos 2 and 3). Right ventricular free wall thickness measured 1.3 to 1.6 mm.

Video 2: Axial stack showing a thin walled right ventricle and severe right atrial dilation.
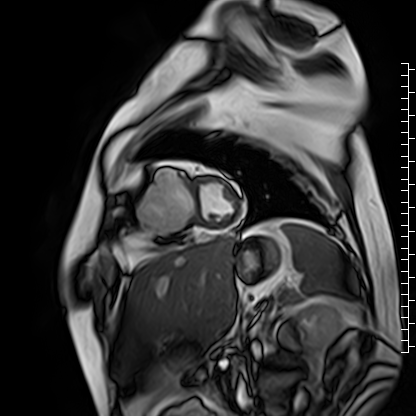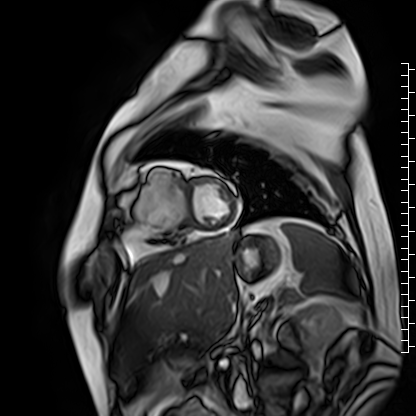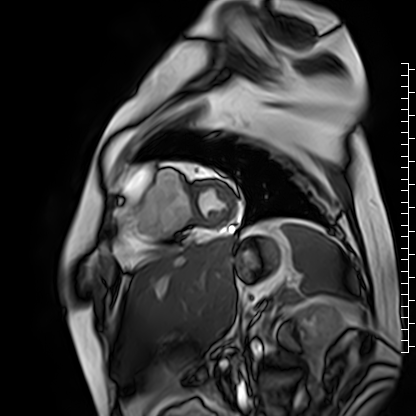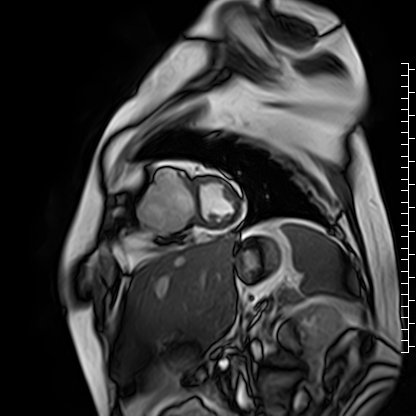
Video 3: Short axis cine image showing paucity of right ventricular myocardium.
Scarce trabeculae were seen in relatively small RV apex. The tricuspid valve was located at normal position (Video 4). Calculated indexed right ventricular volumes were low (RVEDVI: 23 mL/m2 and RVESVI: 17 mL/m2).
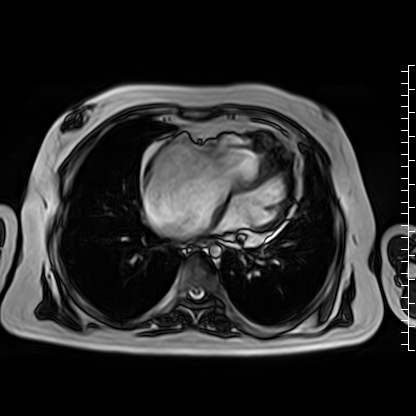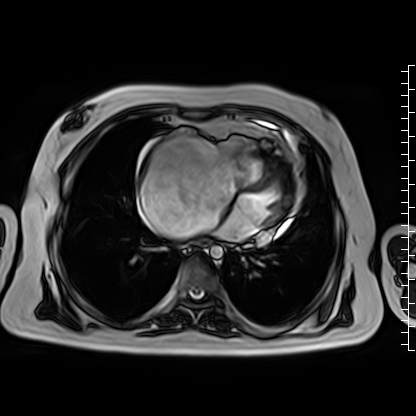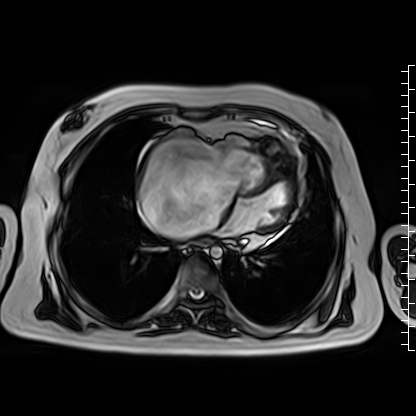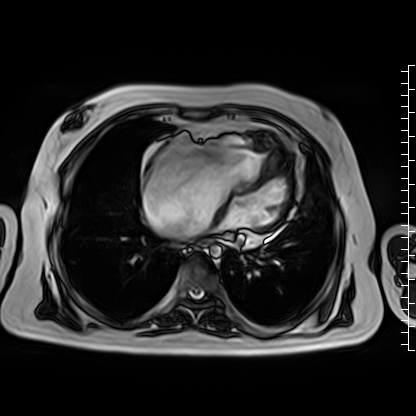
Video 4: Axial cine image demonstrating normal position of the tricuspid valve annulus relative to the mitral valve, thus ruling out Ebstein’s anomaly.
T1 weighted images did not reveal relative hyperintensity in the right ventricle. A corresponding fat saturation sequence was analyzed for any fatty infiltration which was negative (Figure 3).

Figure 3: Fat saturation image showing no overt fat infiltration of the right ventricle.
The right ventricle had moderate to severe systolic dysfunction (RV ejection fraction: 25%). There was diastolic septal flattening suggestive of right ventricular volume overload. Left ventricular systolic function was normal. Delayed enhancement imaging showed patchy late gadolinium enhancement in interventricular septum near inferior hinge point (Video 5). The right atrial wall was thickened, but no right atrial delayed enhancement was detected. There was minimal pericardial effusion with normal pericardial thickness.

Video 5: Delayed enhancement stack.
Conclusion:
Considering all the findings it was evident the patient had right ventricular failure which was confirmed by the CMR. However, the etiology of the RV failure remains in doubt. Uhl anomaly was considered on the basis of scarce RV myocardium in basal region with akinesia of the respective segment, but unlikely as the RV is small and not dilated. There are no known cases of Uhl anomaly with a small RV. Arrhythmogenic cardiomyopathy was considered but in the setting of a small RV, this also seems less likely. The patient does meet one major (Padua) criteria for arrhythmogenic cardiomyopathy with akinesia of the RV and an RVEF <40%.[1] However, the severe right atrial dilation is not a typical feature of arrhythmogenic cardiomyopathy. This would suggest an isolated right ventricular restrictive cardiomyopathy, rarely seen with arrhythmogenic cardiomyopathy. The current working diagnosis is isolated right ventricular restrictive cardiomyopathy versus arrhythmogenic cardiomyopathy. At present, our patient is alive and has class II NYHA heart failure symptoms.
Perspective:
Right ventricular failure is a diagnostic challenge regarding etiology and treatment. The differential diagnosis of isolated right ventricular dysfunction and dilation include arrhythmogenic right ventricular cardiomyopathy, Ebstein anomaly, Uhl anomaly and rarely, isolated RV restrictive cardiomyopathy.
Uhl anomaly is a rare condition described initially by Osler as a thin, “parchment-like” right ventricle and subsequently shown by Henry Uhl to have absence of RV myocardium with apposition of both endocardium and epicardium.[2,5] The underlying mechanism of absence of myocardium in the right ventricle is thought to be apoptosis.[3] Moreover, there is debate whether Uhl anomaly is a separate entity or just a manifestation of broader spectrum of arrhythmogenic cardiomyopathy.
Arrhythmogenic cardiomyopathy is characterized by fatty infiltration in the right ventricular free wall along with variable wall motion abnormalities, arrhythmias, right ventricular and right ventricular outflow tract dilation, and characteristic ECG findings. Revised task force criteria and recently developed Padua criteria are used to diagnose arrhythmogenic cardiomyopathy.[1,4] A definite diagnosis of arrhythmogenic cardiomyopathy requires presence of two major criteria; one major and two minor criteria, or 4 minor criteria. Major criteria include RV dilation/dysfunction, transmural late gadolinium enhancement, T wave inversion in leads V1-3, 500 or more ventricular beats with left bundle branch block morphology, a definite diagnosis in 1st degree relative. Regional areas of myocardial thinning can be noted. Localized micro aneurysms are also a feature of arrhythmogenic cardiomyopathy. Most of the cases have a dilated RV as well. Arrhythmogenic cardiomyopathy with a relatively small RV cavity has not been reported. Presence of one major criteria only classifies as “possible” arrhythmogenic cardiomyopathy.[1] There are no characteristic changes in P waves and usually the right atrium is normal in size.
Ebstein anomaly is characterized by apical displacement of septal leaflet of tricuspid valve and thus atrialization of RV cavity with the anterior leaflet appearing “sail like”. Associated anomalies can include atrial septal defect, ventricular septal defect, pulmonary stenosis, left ventricular outflow tract obstruction, right ventricular outflow tract obstruction, and right atrial dilation. Patients with Ebstein anomaly can be asymptomatic but severe forms can lead to right ventricular failure. An electrocardiogram in Ebstein anomaly typically has large (Himalayan) P waves suggesting the presence of right atrial enlargement.
A rare entity known as isolated right ventricular restrictive cardiomyopathy is also a cause of isolated RV failure. Small RV cavity size and reduced systolic function in turn is characteristic. Isolated RV restrictive cardiomyopathy has been observed with fibroelastosis and is a rare cause of sudden cardiac death.[6] Not many cases of this entity have been reported. Treatment for these patients is challenging. One case report showed development of isolated restrictive cardiomyopathy after chemotherapy for lymphoblastic leukemia. In this case, bidirectional superior cavo-pulmonary shunt with tricuspid valve repair was performed with a good post op result.[7]
Our patient was initially considered with these differentials. Since the tricuspid valve leaflets were structurally normal, Ebstein anomaly was ruled out. Tricuspid insufficiency was likely secondary to annular dilation. There was no evidence of fatty infiltration on CMR however basal RV was thinned and akinetic which does fulfil one major criteria for arrhythmogenic right ventricular cardiomyopathy. No other major or minor criteria was fulfilled. No arrhythmia had been documented. Thin and virtually absent myocardium in the right ventricle was also suggestive of Uhl’s anomaly but a relatively small RV cavity and massively dilated right atrium have not been previously reported in cases of Uhl’s anomaly. Late gadolinium enhancement in cases of Uhl’s anomaly are not frequently described, but a case report showed LGE in interventricular septum.[4] Our case had some late gadolinium enhancement in inferior hinge point which is atypical for Uhl anomaly and arrhythmogenic cardiomyopathy. Lastly, RV restriction remains the most appropriate explanation for all of the findings, although an etiology cannot be identified. It is possible this reflects a congenital disease in our patient rather than an acquired process. His past history of massive pericardial effusion and intermittent cyanosis are clear indicators for the requirement of vigilance regarding overt right ventricular failure and requirement for eventual cardiac transplant although bidirectional cavo-pulmonary shunt also appears an important component of palliation, particularly in low income countries.
Click here to review the images on CloudCMR
Click here to review the images on CloudCMR
References:
- Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, Migliore F, Pilichou K, Rampazzo A, Rigato I, Rizzo S, Thiene G, Anastasakis A, Asimaki A, Bucciarelli-Ducci C, Haugaa KH, Marchlinski FE, Mazzanti A, McKenna WJ, Pantazis A, Pelliccia A, Schmied C, Sharma S, Wichter T, Bauce B, Basso C. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int J Cardiol. 2020 Nov 15;319:106-114. doi: 10.1016/j.ijcard.2020.06.005. Epub 2020 Jun 16. PMID: 32561223.
- Osler WM. 6thed. New York: D. Appleton; 1905. The Principles and Practice of Medicine; p. 280.
- Uhl, H. S. M. (1996). Uhl’s Anomaly revisited. Circulation, 93(8), 1483–1484. https://doi.org/10.1161/01.cir.93.8.1483
- Corrado, D. et al. (2021) Evolving diagnostic criteria for arrhythmogenic cardiomyopathy, Journal of the American Heart Association, 10(18). doi:10.1161/jaha.121.021987.
- Fateh Ali Tipoo Sultan, Babar Hasan, Vincent L. Sorrell, Arash Seratnahaei, UHL’S ANOMALY: YOU’LL RECOGNIZE IT WHEN YOU SEE IT. https://scmr.org/page/COW16
- Chaudhry M, Ashwath ML. Isolated Right-Sided Endomyocardial Fibroelastosis Resulting in Sudden Cardiac Death. JACC Case Rep. 2019 Aug 21;1(2):184-187. doi: 10.1016/j.jaccas.2019.06.018. PMID: 34316781; PMCID: PMC8301532.
- Chiaureli MR, Kovalev DV, Yurlov IA, Minaev AV, Podzolkov VP. Successful One-and-a-Half Ventricle Repair of Right Ventricle Dysfunction Due to Lymphoblastic Leukemia Treatment in a Patient with Restrictive Cardiomyopathy. Am J Case Rep. 2021 Nov 24;22:e933677. doi: 10.12659/AJCR.933677. PMID: 34815376; PMCID: PMC8630555
Case prepared by:
Robert D. Tunks, MD, MHS
Editorial Team, Cases of SCMR
Penn State Health Milton S. Hershey Medical Center







