Puja Sitwala MD, Peter Filev MD, Anurag Sahu MD
Emory University Hospital, Atlanta, Georgia, USA
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical History:
A 60-year-old male volunteer for the homeless presented to the emergency room with a 2-week history of progressively worsening shortness of breath. He had been diagnosed with COVID-19 (coronavirus disease 2019) by a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR in an urgent care facility 4 days prior to presentation. Other associated symptoms included intermittent fever, malaise, and cough. He had noted that his oxygen saturations dropped as low as the 75% on his home pulse oximeter. He had no significant past medical history and was very active prior to this episode.
In the emergency room, he was afebrile, blood pressure was 110/74 mm Hg, heart rate 94 bpm and O2 saturation was 87% on room air. On physical exam there were mild crackles on the lower lung fields bilaterally. Electrocardiogram (Figure 1) showed sinus rhythm with Q waves in the inferior leads (II, III and aVF).
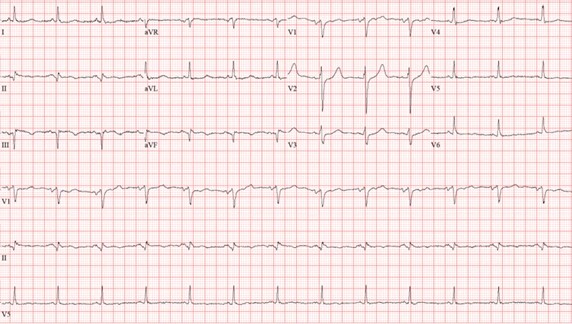
Figure 1: Twelve lead electrocardiogram with sinus rhythm with Q waves in the inferior leads (II, III and aVF).
Pertinent laboratory findings included an elevated white blood cell count 11,300 cells/mcL, elevated D-dimer 15,477 ng/mL, elevated LDH 498 unit/L, elevated BNP (brain natriuretic peptide) 199 pg/mL, minimally elevated troponin 0.06 ng/mL, elevated ferritin 2,342 ng/mL. He was empirically started on full dose heparin.
Chest X-ray (Figure 2) showed multifocal interstitial and patchy alveolar airspace opacities throughout the mid right lower lung and mid left lung consistent with multifocal infection, likely COVID 19.

Figure 2: Chest X-ray with multifocal interstitial and patchy alveolar airspace opacities throughout the mid right lower lung and mid left lung.
CT scan of the chest with contrast (Figure 3) showed a sub-segmental pulmonary embolism of the right lower lobe without evidence of heart strain or pulmonary infarction. Lung parenchymal findings consistent with COVID-19 pneumonia. There was an expansile rounded soft tissue density along the inferior left ventricular border and another mass at the left ventricular apex. A transthoracic echocardiogram with contrast (Movie 1, Figure 4) confirmed cardiomyopathy with multiple wall motion abnormalities as well as the presence of the masses in the left ventricle. A cardiovascular magnetic resonance (CMR) was recommended for further evaluation.
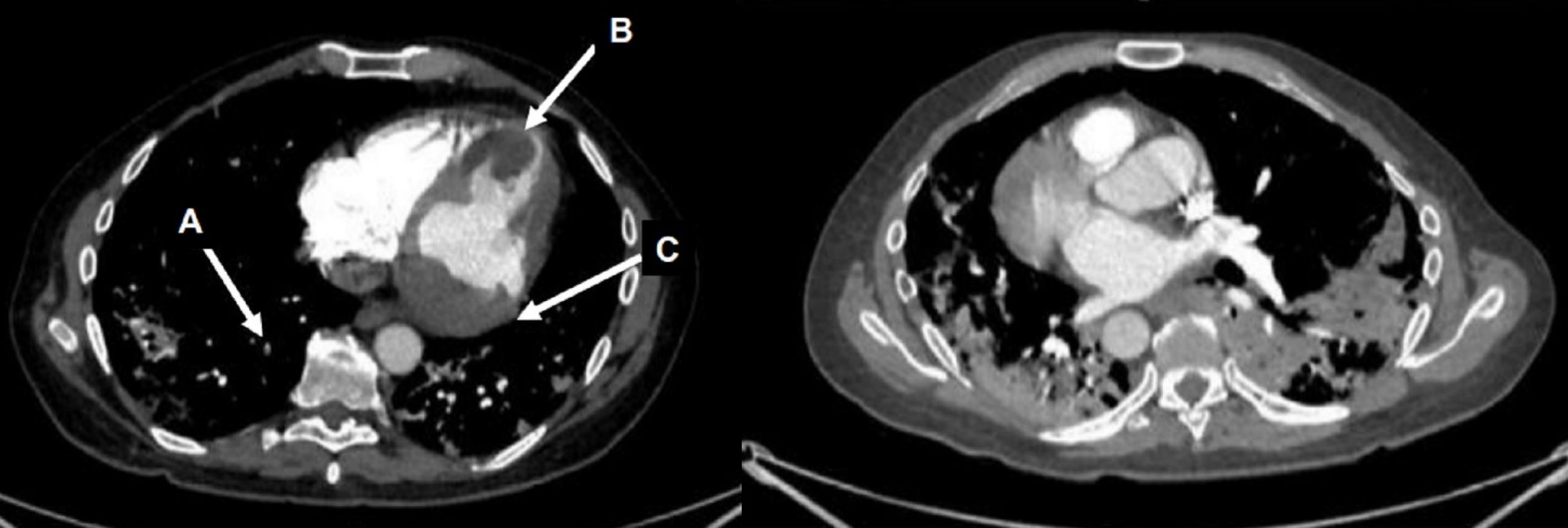
Figure 3: CT scan of the chest with contrast axial view showing a sub-segmental pulmonary embolism of the right lower lobe (A), multifocal pulmonary infiltrates, and a mass near in the inferior border of the heart (B) and in left ventricular apex (C).
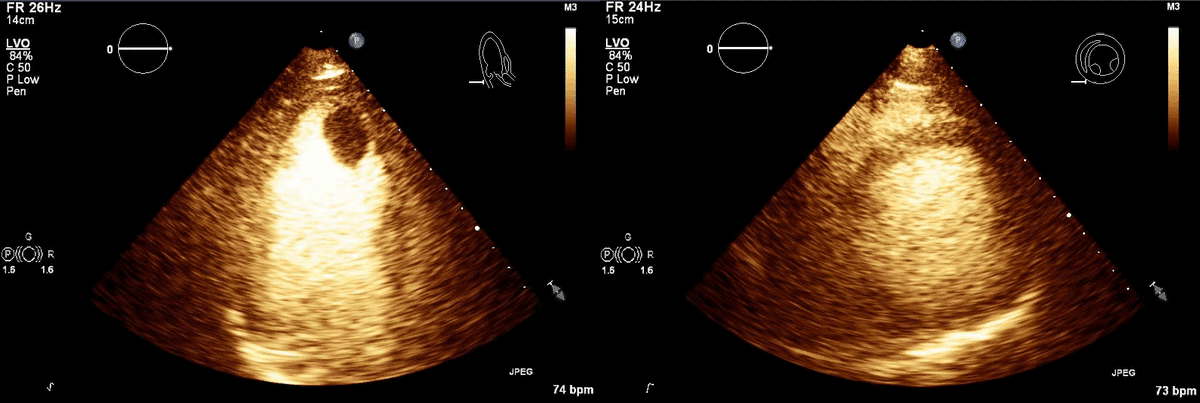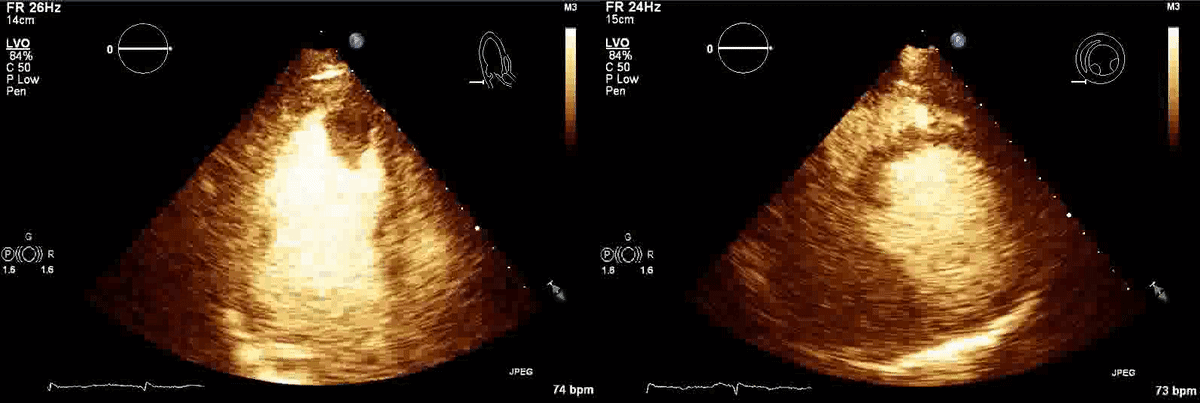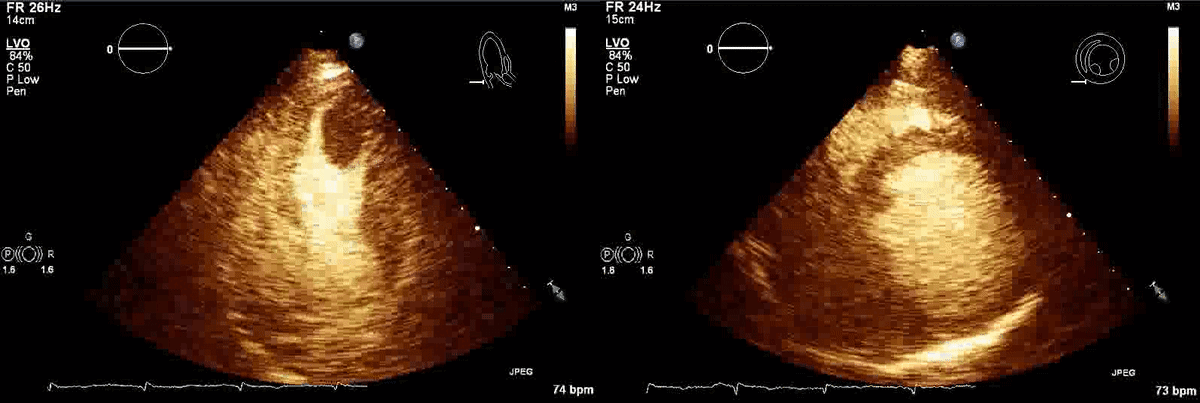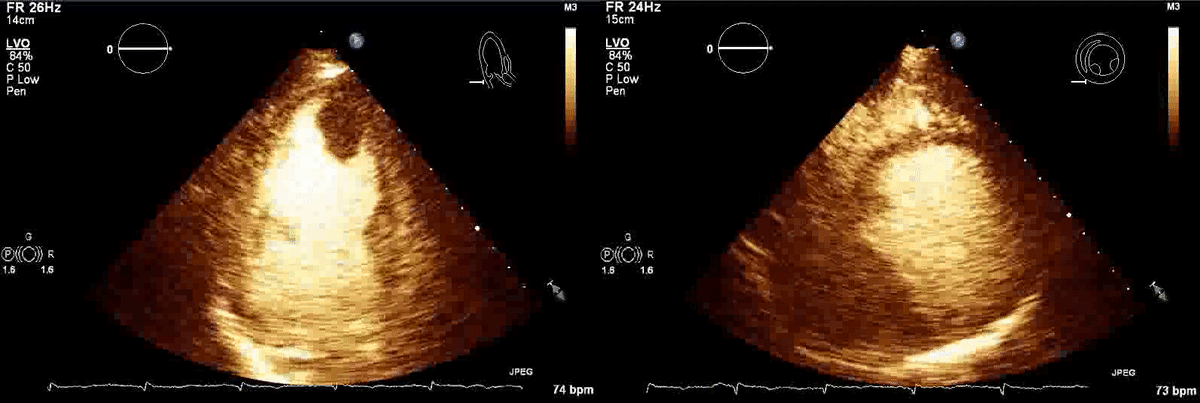
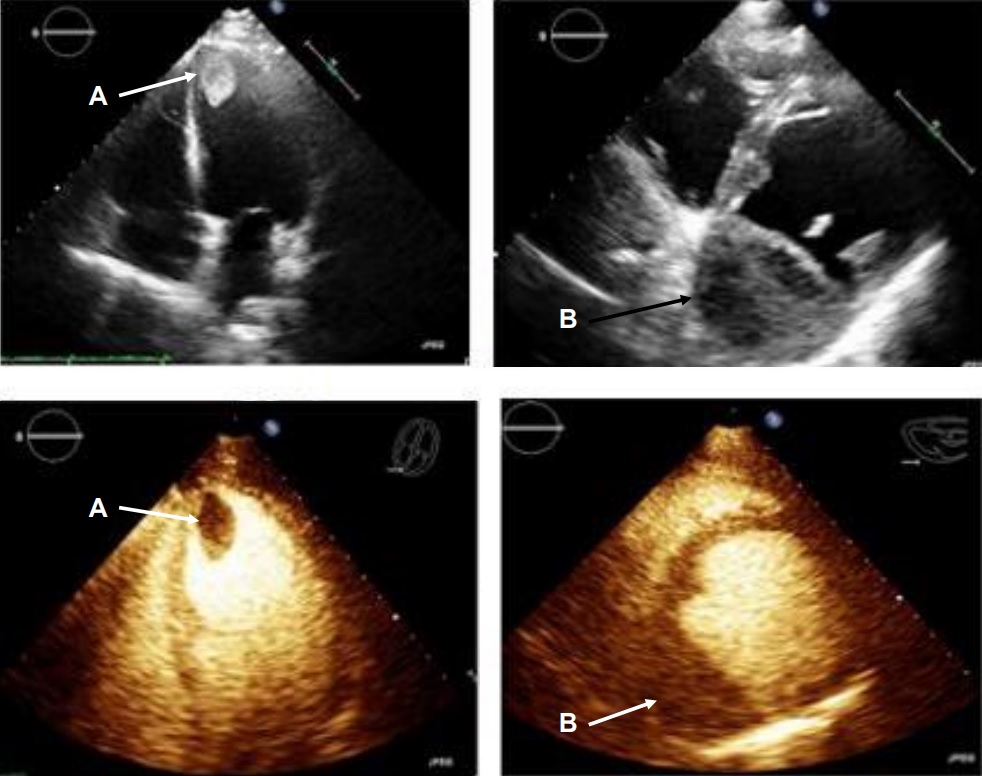
Movie 1, Figure 4: A transthoracic echocardiogram with contrast showed the presence of the masses in the left ventricle apex (A) and inferior wall (B).
CMR Findings:
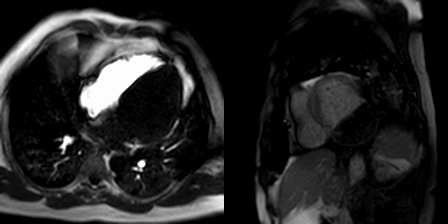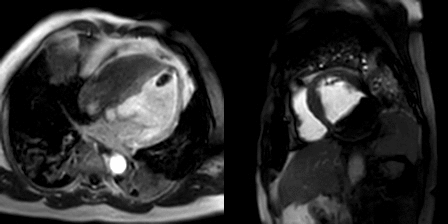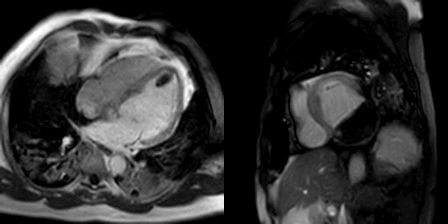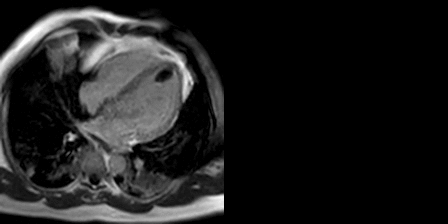
The patient was scanned on a 1.5 Tesla scanner using a ‘cardiac mass’ protocol. The SSFP cine images revealed that the left ventricle was severely dilated with severely reduced systolic function. The left ventricular ejection fraction was calculated at 23%. The basal and mid inferior and inferolateral walls were thin, aneurysmal and occupied by a large mass measuring 6.1 x 4.4 cm. The entire apex was thin, akinetic and there was another mass measuring 3.5 x 1.8 in the left ventricular apex (Movie 2, Figure 5). First pass rest perfusion images showed no uptake in either of the masses (Movie 3, Figure 6).

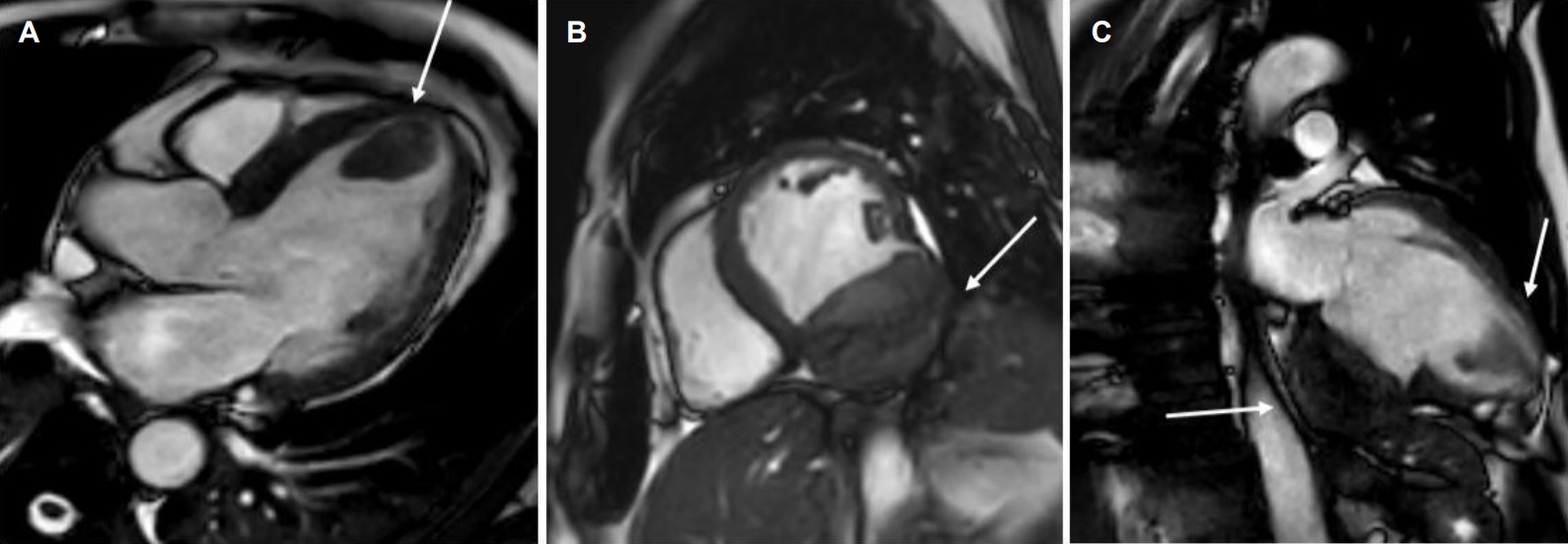
Movie 2, Figure 5: Cine SSFP three chamber (A), short axis (B), and two chamber (C) showing masses (arrows) in the left ventricular apex and inferior wall.
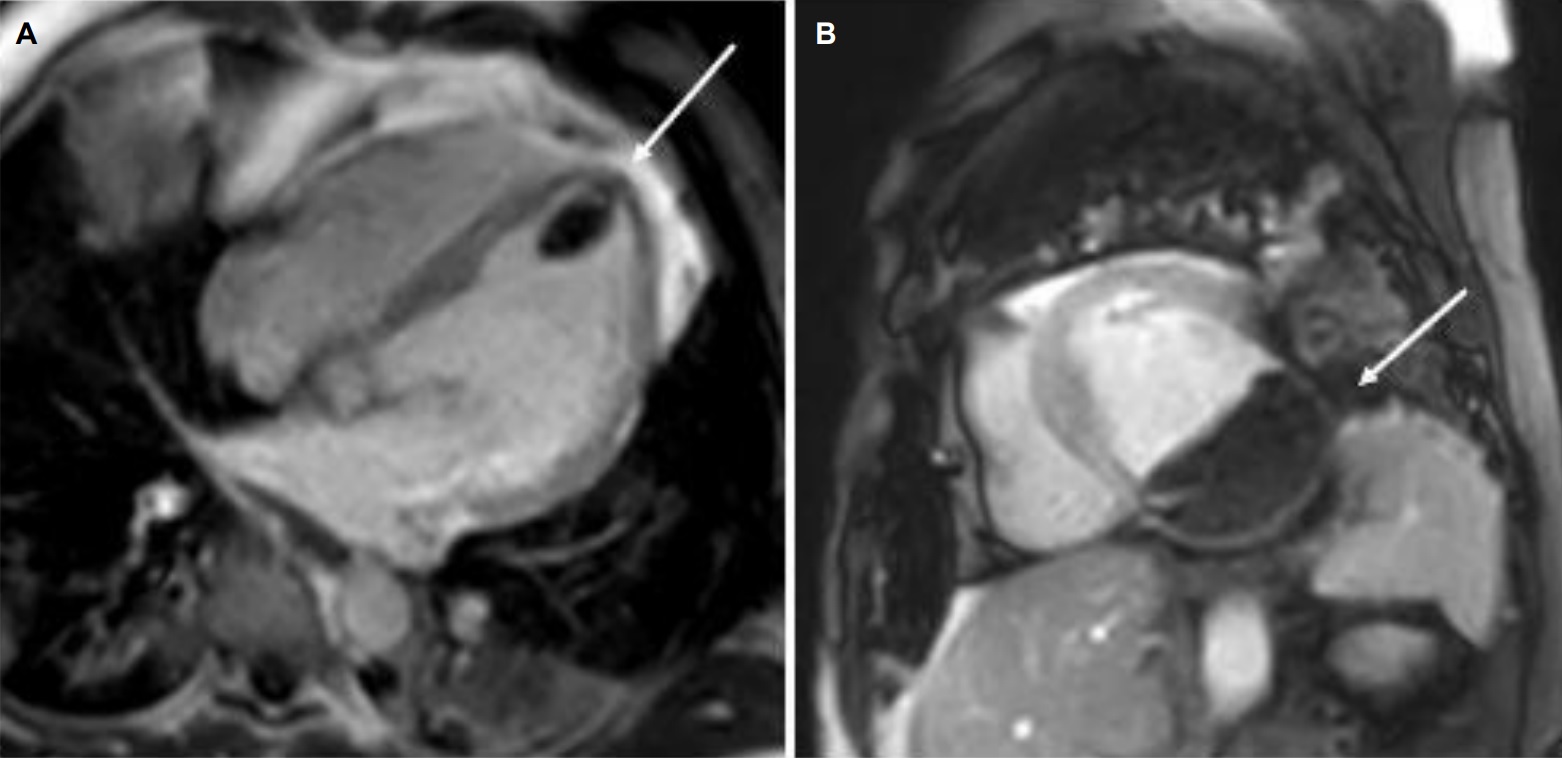
Movie 3, Figure 6: First pass perfusion four chamber (A) and short axis (B) shows no uptake of contrast in either masses (arrows).
The late gadolinium uptake images showed a large transmural infarct in the basal and mid inferior, inferoseptal and inferolateral walls as well as the inferior apical wall. There was also a 50% subendocardial delayed gadolinium enhancement consistent with infarct of the entire left ventricular apex and the right ventricular inferior wall and apex (Figure 7). Another set of delayed gadolinium images were scanned using a prolonged inversion time (i.e. 600 msec) which selectively nulls avascular tissue such as thrombus. This prolonged inversion time renders thrombus black and surrounding myocardium bright as shown in Figure 8. This further corroborated the masses as being thrombi. He was continued on treatment for heart failure, intracardiac thrombi and pulmonary embolism. He was enrolled in a clinical trial for treatment of severe COVID-19 infection.

Figure 7: Late gadolinium enhancement phase sensitive inversion recovery short axis (A), four chamber (B), and two chamber (C) shows a large transmural infarct in the basal and mid inferior, inferoseptal and inferolateral walls as well as the inferior apical wall (white arrows) and 50% subendocardial enhancement consistent with infarct of the entire left ventricular apex and the right ventricular inferior wall and apex (white arrows). No enhancement of the masses (black arrows) of the inferior and apical walls.

Figure 8: Late gadolinium enhancement phase sensitive inversion recovery with long inversion time (600 msec) short axis (A) and three chamber (B) shows thrombus in the inferior wall and apex (arrows).
Conclusion:
In this case, it was thought that the patient likely had COVID-19 related coronary thrombosis of his RCA two weeks prior to presentation that caused an inferior myocardial infarction (MI). His severe COVID-19 infection caused a hypercoagulable state which led to both arterial and venous thromboemboli (the right coronary artery (RCA) was occluded on the detailed review CT pulmonary angiogram and the left sided coronary arteries were well visualized on the CT chest). The CMR facilitated in diagnosing the patient’s infarct and intracardiac thrombi. He was started on goal directed medical therapy for heart failure as well as anticoagulation therapy and discharged on COVID-19 clinical trial medication. He was schedule to see a cardiologist in one-week post discharge at which time they would decide a further treatment plan.
Perspective:
The pathogenesis of hypercoagulability has been commonly explained through Virchow’s triad of stasis, endothelial injury, and a hypercoagulable state. Severe infection with SARS-Co-2 virus causes a proinflammatory state and elevation of levels of several prothrombogenic factors. The infection has also proven to cause direct endothelial cell injury as well as complement mediated damage. There are several studies that showed a higher incidence of venous thromboembolism (both deep vein thrombosis and pulmonary embolism) in ICU and non-ICU patients (though to a lesser extent than ICU patients) infected with SARS-Co-2 virus. There are also several reports of arterial thrombosis including microvascular thrombi, limb ischemia and strokes. It is for these reasons that the American Society of Hematology and the Society of Critical Care Medicine recommend routine aggressive pharmacologic prophylaxis for venous thromboembolism (VTE) unless contraindicated.
Several studies have shown a strong association between risk factors for coronary artery disease such as diabetes, hypertension, prior coronary artery disease and the severity of COVID-19 infection. Severe COVID-19 infections have shown to increase the risk of both Type 1 and type 2 myocardial infarction the latter through hypoxia related illness due to respiratory compromise and primary infection. Troponin elevation is seen in about 10 to 30% of hospitalized COVID-19 patients and was found to be associated with a higher mortality. However, most of the patients presenting with COVID-19 infection, it was found that troponin elevation was not due to acute coronary syndrome (ACS) but secondary to a non-ACS type of myocardial injury such as myocarditis, pericarditis, heart failure, arrythmia related or related to lung injury.
References:
Case Prepared by:
Jason N. Johnson, MD MHS
Editor, COVID-19 Case Collection
Le Bonheur Children’s Hospital, University of Tennessee Health Science Center





