Jafar Alzubi, MD1; Kurian Pannikottu, MD1; Ahmad Jabri, MD1; Vinayak Hedge, MD2; Anmar Kanaa’n, MD2; Joseph Lahorra, MD3.
1 Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH, USA
2 Department of Cardiology, Cleveland Clinic Akron General, Akron, OH, USA
3 Department of Cardiothoracic Surgery, Cleveland Clinic Akron General, Akron, OH, USA
Corresponding Author: Jafar Alzubi, Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH 44307, USA. Email: Jafaralzobi@gmail.com
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical history
A 56 year old male with a past medical history of antiphospholipid syndrome, chronic kidney disease stage III (resulting from a renal infarct from an embolic thrombus), and essential hypertension presented to the emergency department after a near syncopal episode while he was uploading boxes. Upon EMS arrival, he was noted to be in monomorphic ventricular tachycardia (Figure 1) that needed immediate synchronized cardioversion with subsequent resolution of his symptoms.
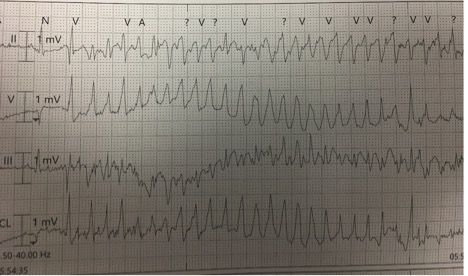
Figure 1. Ventricular tachycardia on presenting ECG
Upon arrival to the emergency room, the patient was hemodynamically stable with benign cardiovascular examination. Resting EKG (Figure 2) showed sinus tachycardia with T wave inversions over the lateral leads.
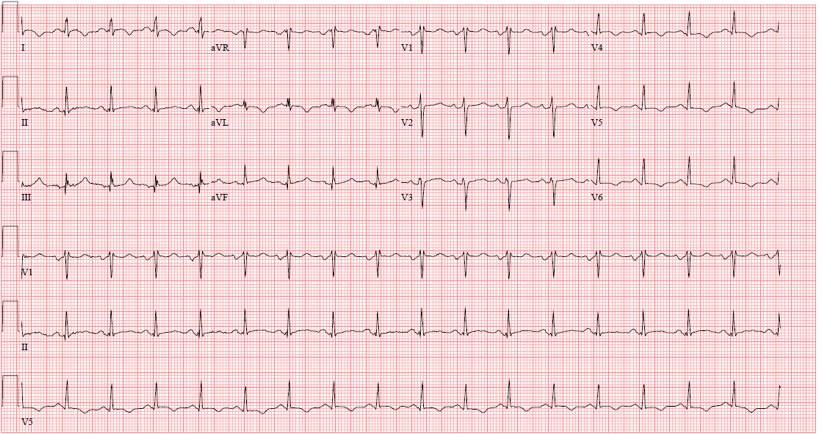
Figure 2. Resting EKG after restoration of sinus rhythm
Lab analysis revealed sub-therapeutic prothrombin time and INR. Initial work up with coronary angiography revealed trivial coronary artery disease and possible congenital coronary anomaly. Transthoracic echocardiogram on the day after admission demonstrated a left ventricular (LV) apical aneurysm with mural thrombus formation. Chest x-ray showed a calcified nodule at the cardiac apex, which correlates to the calcified intramural thrombus. Subsequently, coronary computed tomography angiography (Figure 3 and 4) was obtained revealing a potentially malignant, anomalous right coronary artery (RCA) with left coronary cusp origin just anterior to the origin of the left main coronary artery. This anomalous vessel coursed transmurally between the ascending aorta and pulmonary artery; thus, there is a significant risk for vessel impingement both in the transmural course during systole and between the aorta and the pulmonary artery.
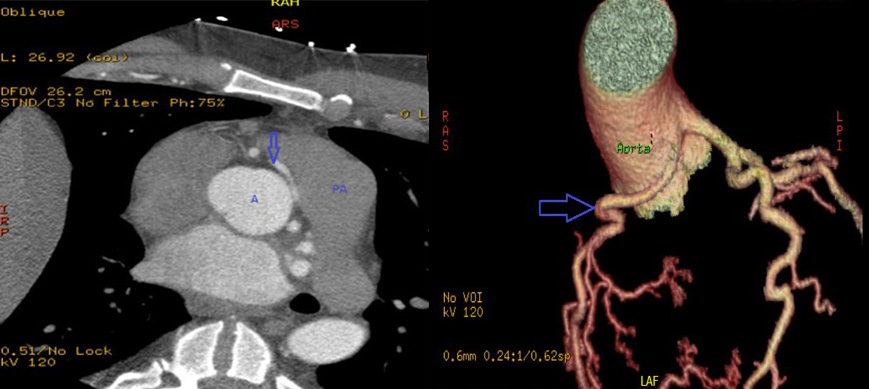
Figures 3 and 4. Transaxial and Volume Rendered CTA showing a potentially malignant, anomalous right coronary artery (RCA) with left coronary cusp origin just anterior to the origin of the left main coronary artery
Echocardiography showed with an ultrasound enhancing agent showed mid-LV hypertrophy and an apical LV aneurysm with an apical mass suspicious for a thrombus.
Finally, cardiac magnetic resonance (CMR) showed mid LV hypertrophy consistent with hypertrophic cardiomyopathy (HCM) and LV apical aneurysm with intramural thrombus with late gadolinium enhanced (LGE) imaging revealed full thickness scarring in the LV apex. Ultimately, an automated implantable cardioverter defibrillator (ICD) was placed, and the patient was sent home with oral anticoagulation and antiarrhythmic therapy. Patient was counseled and recommended to undergo genetic testing for HCM.
Cardiac MRI findings
The CMR steady state free precession (SSFP) cine images revealed a left ventricular ejection fraction of 60% with an apical LV aneurysm measuring 3.9 x 3.2 cm, with moderate hypertrophy of the mid ventricular myocardium measuring 1.6 cm in maximal thickness consistent with HCM. A 1.2 x 1.0 cm lesion was noted within the apical aneurysm, consistent with intramural thrombus(Figures 5,6,7). Also, LGE images revealed near full thickness scarring of the apical segments of LV (Figures 8,9,10).
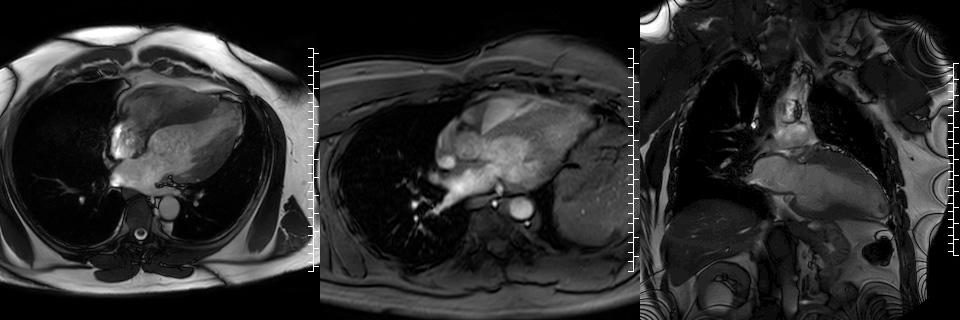
Figures 5,6,7. SSFP Cines in HLA, 3 chamber, and VLA projections demonstrating apical aneurysm and mid LV thickening.
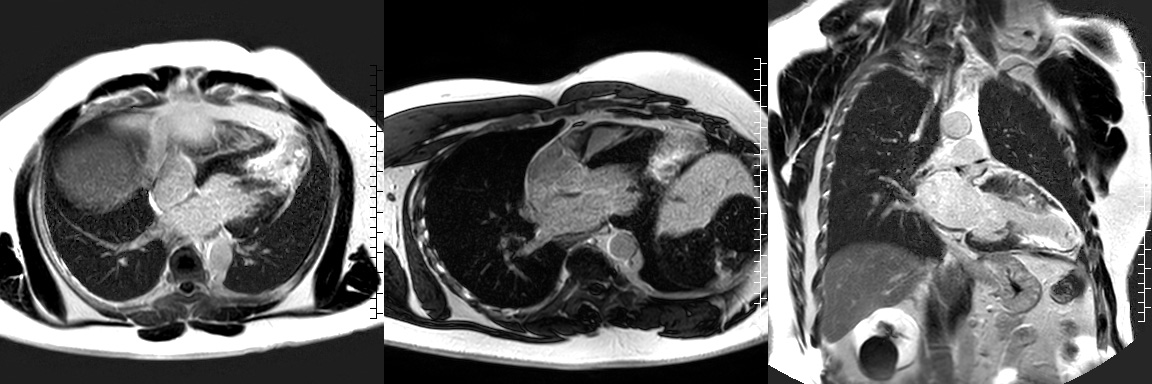
Figures 8,9,10. LGE images of the LV in HLA, 3 chamber, and VLA projection showing dense scar on the mid to apical LV
Conclusion
In conclusion, we report a case of malignant ventricular tachycardia with three plausible causes: anomalous RCA with a potentially malignant course, LV apical scarring with aneurysm formation and HCM. However, the apical scar is the most likely cause of the ventricular tachycardia based on the VT tracing (Figure 1). This case emphasizes the role and importance of CMR in evaluation of new-onset ventricular arrhythmia as it helped determine the most probable source of this life-threatening arrhythmia and saved the patient from undergoing aggressive surgical intervention like: excision and re-implantation of RCA into the appropriate sinus of Valsalva, coronary artery bypass grafting or trans-aortic unroofing of the RCA since such surgical procedures have considerable morbidity and mortality. Hence, it was reasonable to only proceed to ICD implantation in addition to pharmacologic therapy.
Perspective
VT is a life-threatening arrhythmia, and along with ventricular fibrillation, is the most common cause of outpatient sudden cardiac death. Ventricular arrhythmia mostly occurs in setting of structural heart diseases like post-myocardial infarction (most frequently), coronary artery anomalies, dilated cardiomyopathy, hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, and infiltrative diseases like sarcoidosis in addition to non-structural heart diseases like in long QT syndromes. Hence, in patients with new-onset VT, it is vital to pinpoint the source of the aberrant rhythm to prevent the recurrence of potentially life-threatening arrhythmias. As was true in this case, multi-modality imaging plays a major role in evaluation and management of this arrhythmia.
The following will be a discussion of the three potential origins:
1) Anomalous RCA
While most of coronary artery anomalies (CAA) are not associated with myocardial ischemia and clinical events, some anomalies can potentially result in myocardial ischemia depending on the location and course of the anomaly; thus, they can manifest with angina pectoris, syncope, myocardial infarction and ventricular tachycardia; however, CAAs are considered an under-diagnosed cause of sudden cardiac death (SCD) in young athletes (1).
Anomalies of RCA include ectopic origin from the right sinus of Valsalva, posterior sinus of Valsalva and left sinus of Valsalva (LSV). Our patient has ectopic RCA originating from LSV which has an incidence rate of 0.03% to 0.92% on coronary angiograms (2). In our patient, the long intramural course of the anomalous RCA in addition to its inter-arterial course between the aorta and pulmonary artery can potentially result in compression especially during exercise and decreased blood flow to myocardium potentiating ischemic ventricular arrhythmia.
Coronary anomalies can be seen on echocardiography, angiography, coronary CT and cardiac MRI with coronary CT being the diagnostic standard, this is due to the high spatial resolution and excellent acquisition time offered by Coronary CT (3).
2) Left ventricular apical scar
Ventricular scar or fibrosis most commonly forms as a consequence of myocardial infarction, but it can be also caused by non-ischemic insults like myocarditis. These scars are composed of fibrous tissue with intervening cardiomyocytes, and because fibrous tissue is unexcitable, it represents a substrate for re-entry circuits leading to ventricular arrhythmia. As was true in this case, LGE in CMR is the gold-standard imaging for diagnosis of myocardial scar. Normal and viable myocardium appears black on LGE imaging while myocardial scar appears bright or enhanced. Myocardial scar detected on imaging is considered a strong predictor for cardiac death and major adverse cardiac events (4).
In our patient, the apical scarring was thought to be related to either HCM or less likely thrombotic or embolic event causing infarction in the distal LAD territory given patient’s history of anti-phospholipid syndrome in setting of sub-therapeutic anticoagulation. It is thus important that the patient be continued on anticoagulation as he would be at risk for further embolic phenomenon.
3) Hypertrophic cardiomyopathy
HCM is the most common cause of SCD in young athletes. It is mostly asymmetric with most cases having involvement of the basal inter-ventricular septum. However, hypertrophy can exclusively involve the apex, or mid-portion or posterior wall of the LV (5).
Diagnosis of HCM can be made by demonstrating a wall thickness >15 mm in one more LV myocardial segments by any cardiac imaging modality, not explained solely by loading conditions (6). Although the patient in this case has chronic kidney disease and hypertension, neither of these diseases can explain the left ventricular hypertrophy demonstrated on the patient’s imaging as it is focal and asymmetrical (involving mid-segment of the inter-ventricular septum).
Although echocardiogram is considered a good assessment tool for LV wall thickness and left ventricular outflow tract pressure gradient, CMR should be considered for accurate assessment of LV wall thickness in patients with poorly visualized LV regions on echocardiogram, and particularly in HCM cases that are confined to one or two segments of LV like apical or anterolateral variants because of its superior characteristics including lack of attenuation, great spatial resolution in addition to its ability to image in any plane (6). Also, LGE by CMR plays an important prognostic role in predicting adverse cardiovascular events among HCM patients (7).
1. Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000 May;35(6):1493-501.
2. Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 2016 Jun 28;8(6):537-55.
Please click to view the case on CloudCMR
Case prepared by:
Dr Ashish Aneja
Associate Editor SCMR Case of the Week







