Case from: Jaspreet Singh, MD; Scott Klewer, MD; Aiden Abidov, MD, PhD
Institute: The University of Arizona, Tucson, Arizona
Clinical history: We present a case of a 27 year old Native American male with a past medical history of complex congenitally corrected transposition of great arteries (CCTGA) with associated double outlet right ventricle (DORV), dextrocardia, subpulmonary stenosis, subpulmonary VSD and PDA. He also had a congenital solitary kidney. The patient underwent resection of subpulmonary stenosis, VSD patch closure, and PDA ligation at six years of age and was followed and treated with standard medical therapy for congestive heart failure at an outside hospital after the surgery.
The patient presented to our congenital cardiology clinic with fatigue, decreased exercise tolerance, worsening exertional shortness of breath, and intermittent lower extremity edema. His ECG was performed with deliberate lead reversal and showed normal sinus rhythm with RBBB, RAD, and R wave dominance in lead V1, left atrial enlargement with bifid p wave, and ST-T wave changes consistent with systemic ‘morphological’ right ventricular strain (Figure 1).
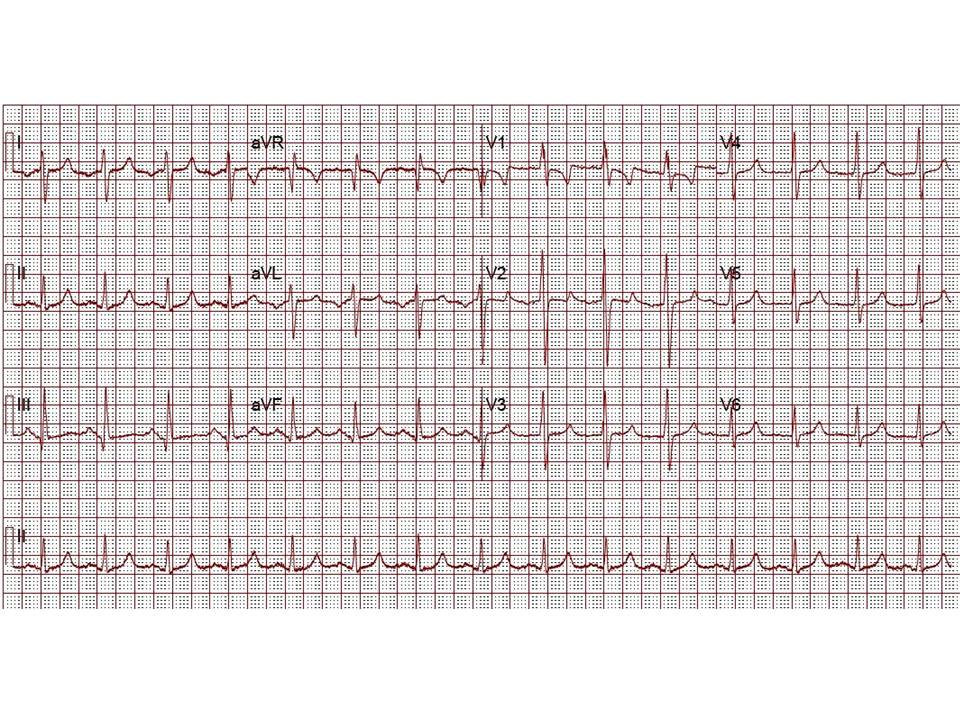
Figure 1: ECG
A transthoracic echocardiogram (TTE) revealed situs solitus, a malposed heart to the right, atrioventricular (AV) discordance, dilated systemic ‘morphological’ right ventricle (RV) with severely decreased systolic function, severe tricuspid regurgitation (TR) (Video clip 1), L- transposition of great arteries, severe pulmonic regurgitation (PR), and mild mitral regurgitation (MR).
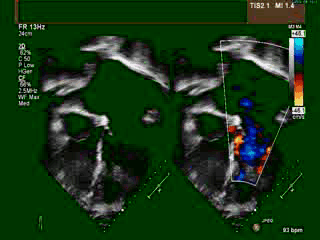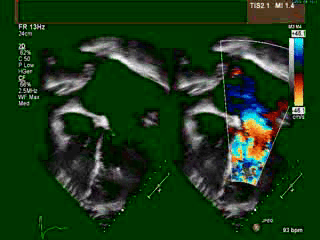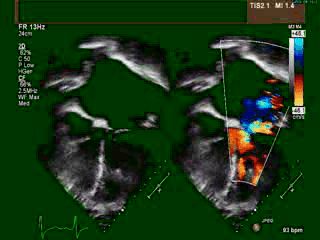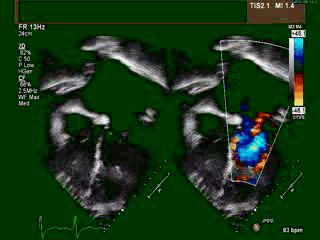
Video Clip 1: A 4-chamber view on transthoracic echocardiography with and without a color Doppler signal showing the features of dextrocardia and CCTGA with hypertrophied ‘morphological’ RV on the left side, as demonstrated by the presence of a moderator band and apically displaced tricuspid valve. Also seen is severely enlarged left atrium. The apex of ‘morphological’ LV points to the right. Color Doppler imaging demonstrated the presence of severe tricuspid regurgitation.
He was referred for Cardiac MRI at this time as a part of cardiac transplant evaluation due to his worsening clinical status on standard medical therapy for congestive heart failure.
CMR Findings
CMR imaging revealed abdominal and atrial situs solitus with dextrocardia, features of DORV, and L-transposition of great arteries with aorta anterior and to the left of pulmonary artery (Figures 2, 3, and 4).
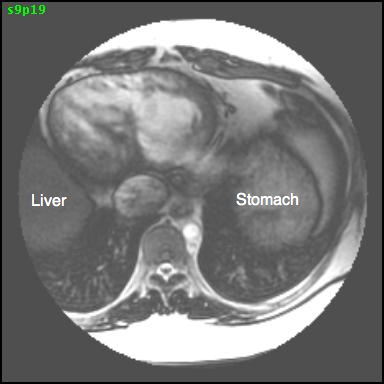
Figure 2: Axial SSFP image showing the presence of abdominal situs solitus with the liver on the right and stomach on the left.
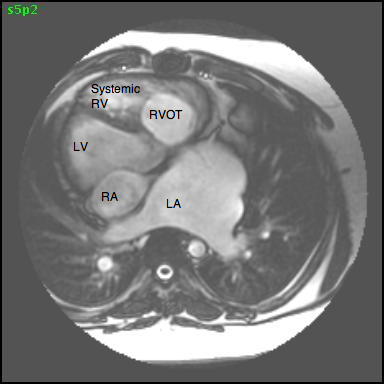
Figure 3: Transaxial 4-chamber SSFP image showing atrial situs solitus and dextrocardia with the apex of the ‘anatomical LV’ pointing to the right (LA= left atrium, LV= morphological left ventricle, RA= right atrium, RV= morphological right ventricle, RVOT= right ventricular outflow tract).
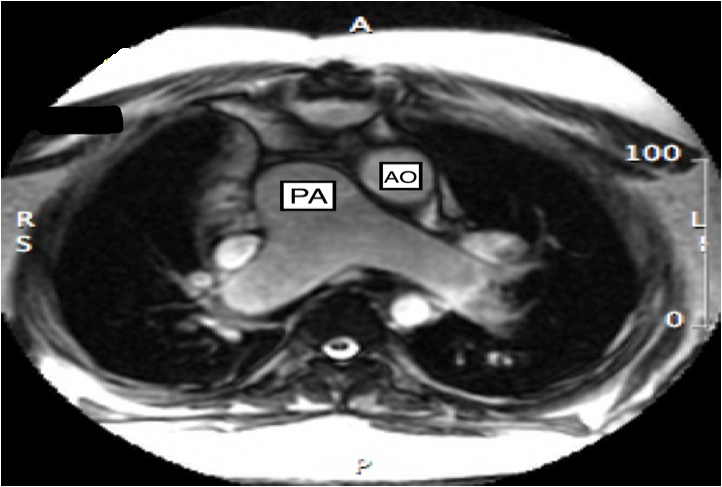
Figure 4 Transaxial SSFP image showing features of CCTGA with malposition of great arteries with aorta anterior and to the left of pulmonary artery (AO = aorta, PA = pulmonary artery)
Conal tissue was noted separating the pulmonic and mitral valves, consistent with DORV (Figure 5)
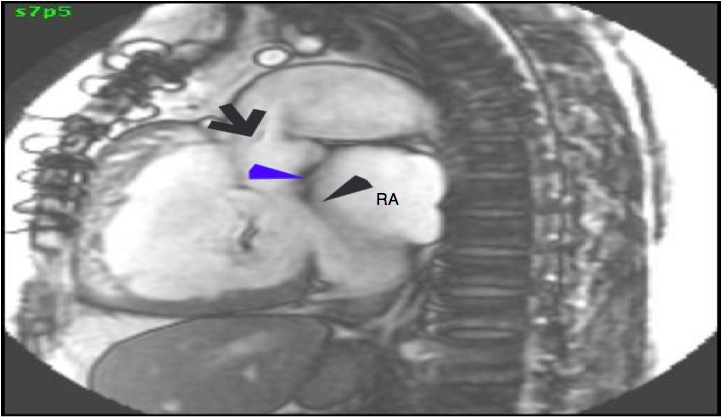
Figure 5: A sagittal SSFP image showing the location of pulmonic valve, mitral valve, and conus tissue separating the pulmonic and mitral valve. Black arrow pointing towards location of the pulmonic valve leaflets, blue arrowhead showing conus muscular tissue, and black arrow-head pointing towards the mitral valve leaflets (RA= right atrium).
The aorta originated from the left sided ‘morphologic’ RV (systemic ventricle), with left sided aortic arch (Video clip 2). The main pulmonary artery (PA) originated from the right-sided morphologic left ventricle (LV) and demonstrated severe pulmonic regurgitation (Video clips 3 and 4).
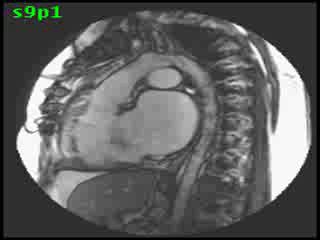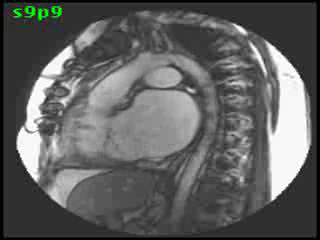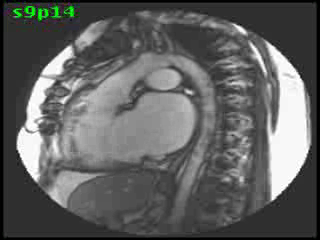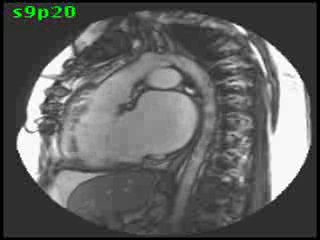
Video clip 2: An oblique sagittal SSFP view showing aorta originating from the left sided morphological RV (systemic ventricle), with left sided aortic arch and enlarged aortic root.
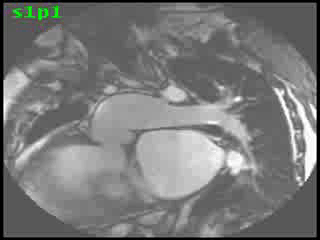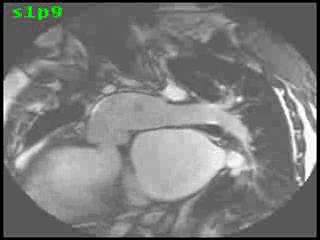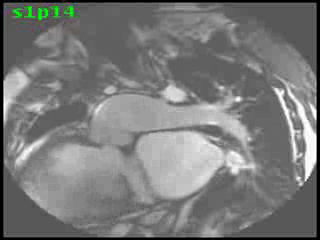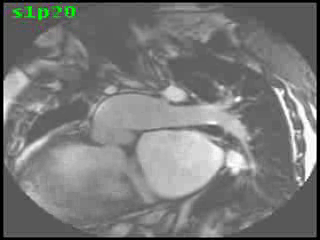
Video Clips 3 and 4: The main pulmonary artery appeared to originate from the right-sided morphologic LV and showed severe pulmonic regurgitation as seen on oblique sagittal views on SSFP imaging.
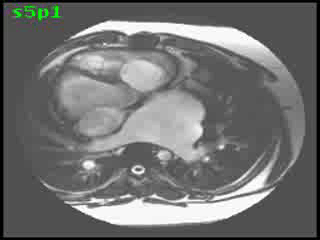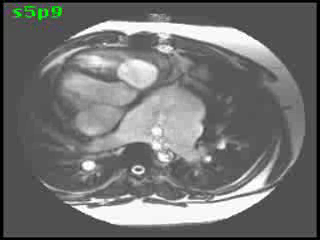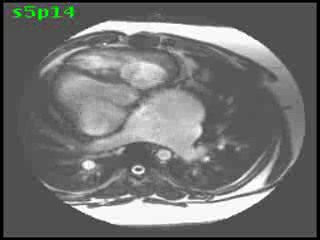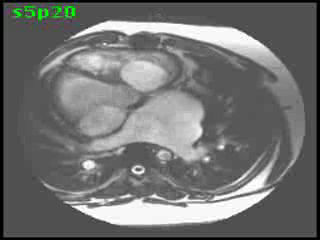
Morphological RV (systemic ventricle) showed hypertrophy and enlargement with global hypokinesis and reduced RV ejection fraction of 30%. The morphological LV demonstrated moderate enlargement and severely reduced systolic function with LV ejection fraction of 22% (Video clip 5). Severe TR and mild MR were present as seen on axial 4-chamber SSFP view (Video clip 6)
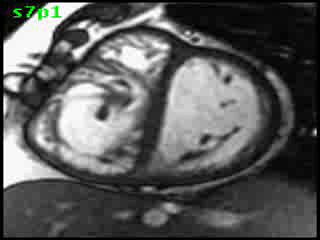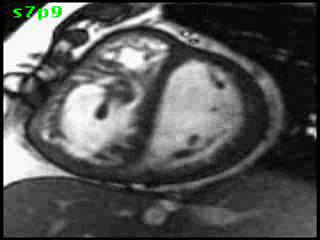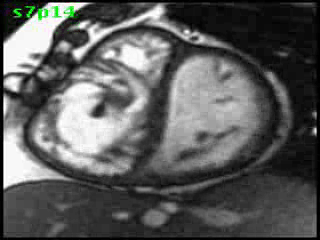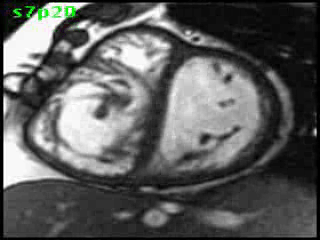
Video clip 5: An oblique SSFP short axis view showing hypertrophy and enlargement of the morphological RV (systemic ventricle) with global hypokinesia and reduced RVEF 30%. Morphological LV showed moderate enlargement with a severely reduced LVEF of 22%
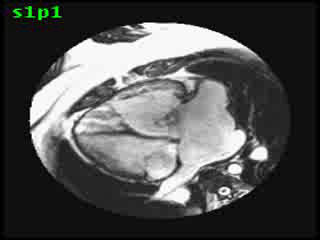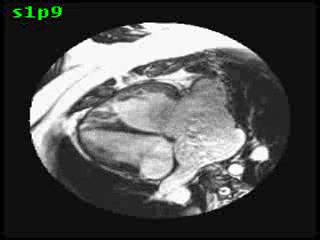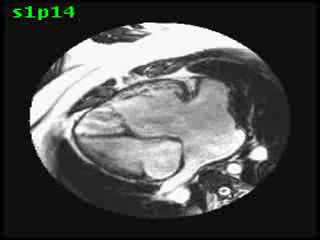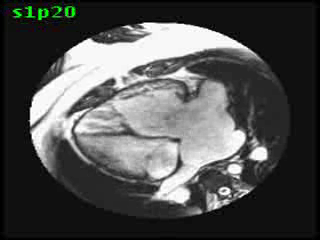
Video clip 5: An oblique SSFP short axis view showing hypertrophy and enlargement of the morphological RV (systemic ventricle) with global hypokinesia and reduced RVEF 30%. Morphological LV showed moderate enlargement with a severely reduced LVEF of 22%
Quantitative flow analysis on velocity mapping of the pulmonary artery flow revealed severe pulmonic regurgitation (Figure 6):
1) Forward flow= 9529 ml/min; 2) Regurgitant flow= 3697 ml/min; and 3) Regurgitant fraction= 38%.
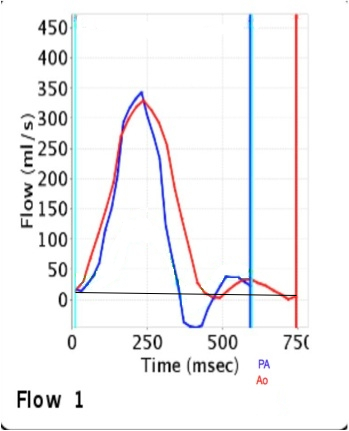
Figure 6: Flow velocity mapping on phase contrast imaging confirmed the presence of severe pulmonic regurgitation with a regurgitant fraction of 38%. QP: QS was 0.93 demonstrating the absence of significant residual VSD shunt (PA= pulmonary artery, Ao= aorta).
Delayed enhancement imaging was aborted as the patient developed severe nausea after administration of intravenous gadolinium and could not tolerate additional scan time.
Subsequently, the patient also underwent right and left heart catheterization, which revealed an anterior coronary system that coursed to the right and bifurcated into right-sided LAD and circumflex. The posterior coronary artery coursed leftward and gave rise to a right coronary system. There was no obstructive disease noted in the coronaries. Right heart catheterization revealed severe pulmonary hypertension with mean right atrial pressure of 17 mm Hg, pulmonary ventricular pressure (morphological LV) of 78/8/18 mm Hg, pulmonary artery pressure of 75/45/59 mm Hg, mean pulmonary capillary wedge pressure of 32 mm Hg and pulmonary vascular resistance (calculated) equal to 5.1 WU. Retrograde left heart catheterization revealed systemic ventricular (morphologic RV) pressure of 104/5 mm Hg, ascending aortic pressure of 93/64 mm Hg, and cardiac output of 5.3 l/min.
Conclusion: CMR confirmed the findings of transthoracic echocardiography, both qualitatively and quantitatively by showing severely decreased biventricular systolic function with elevated systemic morphologic RV end diastolic volume of 131ml/m2 and severe systemic tricuspid valvular regurgitation. It also showed severe pulmonic regurgitation as a complication of repair of subpulmonary stenosis, which was confirmed by flow velocity mapping. CMR ruled out the presence of any residual VSD (QP: QS= 0.93) and subpulmonary stenosis. It also ruled out the possibility of a tricuspid valve or a modified double switch repair by confirming and quantifying the severe degree of biventricular failure. Based on the CMR findings, the patient was referred to cardiothoracic surgery for possible listing for cardiac transplant.
Perspective: CCTGA is a rare anomaly, with an incidence ranging from 0.03 per 1000 live births accounting for approximately 0.05% of congenital heart malformations.1 Associated anomalies include tricuspid valve abnormalities (e.g., Ebstein’s anomaly), VSD, subvalvular and valvular pulmonary stenosis, RV hypoplasia, dextrocardia, and conduction abnormalities, including complete heart block.2 Tricuspid regurgitation and atrioventricular conduction abnormalities are well known to progress and are significant risk factors for survival. With regard to regurgitation, increasing degrees of right ventricular failure commonly accompany this problem as seen in our patient.3
There are various surgical approaches to the management of CCTGA. A recent technique for complex CCTGA (CCTGA+ VSD/PS) management would include creation of a hemi-mustard atrial baffle to direct IVC flow across the tricuspid valve, a bidirectional Glenn (SVC-PA) anastomosis to unload the post-op RV along with a Rastelli intraventricular baffle to connect the morphologic LV with the aorta. The MPA is oversewn and RV to PA continuity is established by an RV to PA conduit. This surgical approach results in the left ventricle functioning as the systemic ventricle. Although long term outcomes of the modified double switch for complex CCTGA are not know, intermediate results are favorable.4
CMR plays an important role in follow up by detecting changes in the ventricular volume and function over time in patients with CCTGA with failure of the systemic morphological RV.5 As depicted in our case, the patient developed a severe decrease of the RV function (systemic ventricle), with clinical symptoms of CHF and was referred for cardiac transplantation. CMR also plays a role in the post-operative follow up of patients with CCTGA to screen for surgical complications and sequelae. In our case, the patient developed severe pulmonic regurgitation and LV dysfunction, likely as sequelae of VSD repair.
As compared to cardiac CT, CMR can be used in serial studies to evaluate complex congenital heart disease without exposing young patients to repeated doses of ionizing radiation and contrast.
In patients with advanced complex CHD, late gadolinium enhancement can be used to detect myocardial fibrosis, which has been shown to be associated with right ventricular dysfunction. However, this could not be performed in our patient due to the development of severe nausea after administration of intravenous gadolinium.6
References:
1. Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill CA, Perry LW, Hepner SI, Downing JW. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 121(1):31-6
2. Warnes CA. Transposition of the great arteries. Circulation 2006;114:2699-2709
3. Dyck JD, Atallah J: Congenitally Corrected Transposition of the Great Arteries. In: Moss and Adams’ Heart Disease In Infants, Children, and Adolescents Including The Fetus and Young Adult, seventh edition, Philadelphia: Lippincot Williams & Wilkins, 2008, pp. 1087-1100.
4. Malhotra SP, Reddy VM, Qiu M, Pirolli TJ, Barboza L, Reinhartz O, Hanley FL. The hemi-Mustard/bidirectional Glenn atrial switch procedure in the double-switch operation for congenitally corrected transposition of the great arteries: rationale and midterm results. J Thorac Cardiovasc Surg 2011 Jan;141(1):162-70
5. Bremerich J, Wyttenbach R, Buser P T, Higgins CB: Cardiovascular Magnetic Resonance in Complex Congenital Heart Disease. In: Warren J. Manning, MD, Dudley J. Pennell, MD, Cardiovascular Magnetic Resonance second edition, Philadelphia: Saunders, 2010, pp. 408-419.
6. Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, Gatzoulis MA, Kilner PJ. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation 2005 Apr 26;111(16):2091-8
COTW handling editor: Sylvia Chen
Have your say: What do you think? Latest posts on this topic from the forum







