Case from: Sharmin Basher1, Mark Lawson1, Rashid Ahmad1, Rebecca Hung1, William Bradham1
Institute: 1 Department of Cardiology, Vanderbilt University Medical Center, Nashville, TN
Clinical history: A 70 year old man with hepatitis B, solitary kidney and remote coronary artery bypass grafting from 17 years prior presented with an inferior myocardial infarction. Coronary angiography demonstrated advanced disease necessitating repeat bypass surgery. Surgery was planned after a waiting period to allow for recovery from the acute myocardial infarction; however, the patient developed unstable angina prompting emergent surgical revascularization. A month later he was readmitted with NYHA Class IV heart failure symptoms and was placed on inotropes. Transthoracic echocardiogram (TTE) revealed severely depressed left ventricular systolic function and an extensive inferolateral aneurysm measuring 86mm from the neck to furthest wall of the abnormality.
CMR Findings: CMR confirmed a large lateral wall true left ventricular (LV) aneurysm measuring 61mm (Figure1; Movie 1,2). The LV ejection fraction (LVEF) including the aneurysm was 8%. The anterior and inferior walls, and the septum, in contrast, contracted normally while the lateral wall was dyskinetic.
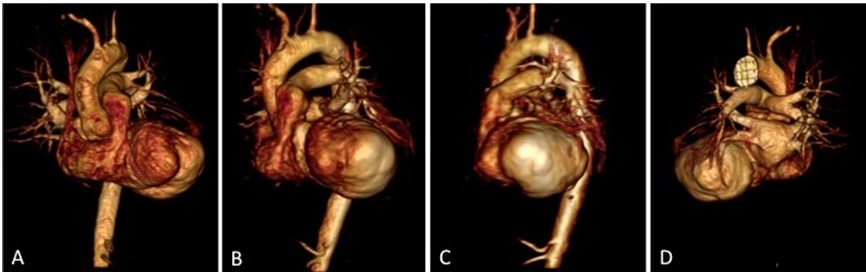
Figure 1: 3D Reconstructed Views of Left Ventricle and Aneurysm Before Surgical Intervention: (A) Coronal View, (B) LAO View, (C) Sagittal View, (D) Posterior View

Movie 1: 3D Reconstructed View of Left Ventricle and Aneurysm Before Surgical Intervention.

Movie 2: Cine of CMR LV Outflow Tract Demonstrating Pre-surgical LV Aneurysm Extent
The anterior, inferior and septal walls demonstrated no late gadolinium enhancement, indicating viable myocardium, while the lateral wall demonstrated transmural late gadolinium enhancement (Figure 2A,B). Surgical intervention was postponed for 6 weeks due to myocardial friability and poor tissue integrity immediately following the recent infarct.

Figure 2: CMR LV Outflow Tract Images (A) Pre-surgical aneurysm extent, (B) Pre-surgical late Gd enhancement of inferolateral wall lined by thrombus.
The aneurysm, which extended to the posterior mitral valve annulus, was carefully resected to avoid damaging the mitral valve apparatus. Following aneurysmectomy, the left ventricle assumed a mostly ellipsoidal geometry with a residual basal aneurysmal “pocket” which could not be resected due to proximity to the atrioventricular groove. Follow-up CMR demonstrated reduction of LV end diastolic volume from 457 mL to 285 mL and improvement in LVEF to 22% (Movie 3; Figure 3 C,D). Since surgery the patient received an implantable cardiac defibrillator and his performance has improved to NYHA Class II with no further hospitalizations for heart failure.
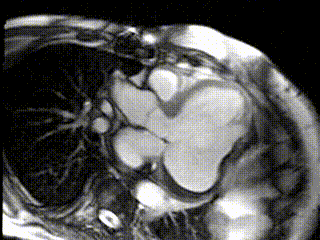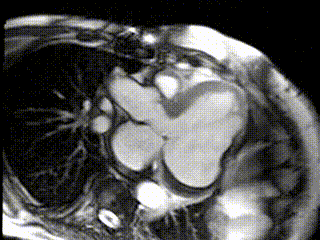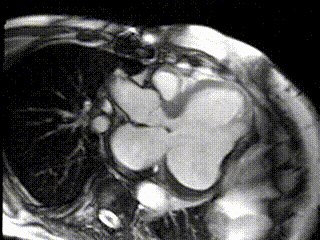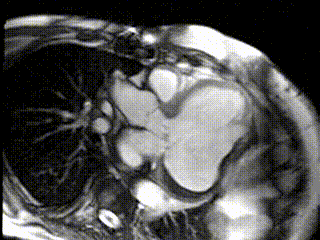
Movie 3: Cine of CMR LV Outflow Tract Demonstrating Post-surgical LV Size and Function.
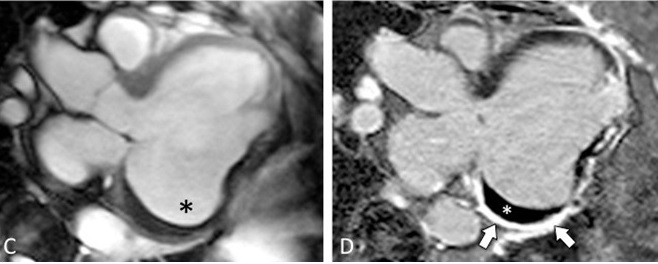
Figure 3: CMR LV Outflow Tract Images (C) Post-surgical residual aneurysm pocket (black *), (D) Post-surgical late Gd enhancement of residual aneurysm of inferolateral wall (arrows) and thrombus (white *)
Conclusion: The differentiation of a true aneurysm from a pseudoaneurysm is critical to the management strategy. Pseudoaneurysms develop secondary to ventricular free wall rupture, which are contained by overlying pericardium or scar tissue without endocardium involvement; in contrast to true aneurysms, which are contained by myocardium [1]. Pseudoaneurysms are more likely to rupture and early identification is paramount. In the absence of indications for surgery, true aneurysms usually do not rupture in their chronic stage and can be medically managed.
TTE is typically the initial imaging modality utilized to visualize the hemodynamic connection between the aneurysm and the LV cavity. Visualization of the left ventricular apex can be limited on TTE, which is the usual location of the majority of aneurysms. CMR is an imaging modality that plays an important role in differentiating a true aneurysm from a pseudoaneurysm since CMR has sufficient resolution to distinguish myocardium, pericardium, and mural thrombus, which are difficult to distinguish by contrast ventriculography [2]. Late gadolinium enhancement localizes to infarcted myocardium and provides quantification of infarct extent.
Perspective: Although there are a variety of imaging modalities that can be utilized to establish the presence of a left ventricular aneurysm, CMR provides a comprehensive evaluation of the aneurysm. Transthoracic echocardiography is often limited by suboptimal windows and imaging planes. Angiography is the most reliable method leading to definitive diagnosis, however it is an invasive procedure unlike CMR and incurs the risk of dislodging thrombus from within the aneurysm. CMR is therefore a useful imaging modality to identify left ventricular aneurysm and morphology, differentiate between true and false ventricular aneurysms, identify thrombus, determine myocardial viability, and assess left ventricular function following surgical intervention [3].
References:
1. Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm: a review of the literature. Chest 1997;111:1403-9.
2. Kumbasar B, Wu KC, Kamel IR, Lima JA, Bluemke DA. Left Ventricular True Aneurysm: Diagnosis of Myocardial Viability Shown on MR Imaging. Am J Roentgenology. 2002; 179: 472-474.
3. Heatlie GJ, Mohiaddin R. Left ventricular aneurysm: comprehensive assessment of morphology, structure and thrombus using cardiovascular magnetic resonance. Clin Radiol. 2005 Jun;60(6):687-92.
COTW handling editor: Kevin Steel, MD
Have your say: What do you think? Latest posts on this topic from the forum







