Tomas Zaremba1*, Katrine Mikala Müllertz2, Steen Hvitfeldt Poulsen2, Henning Mølgaard2, Won Yong Kim2
1Department of Cardiology, Aalborg University Hospital, Hobrovej 18-22, DK-9100 Aalborg, Denmark
2Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, DK-8200 Aarhus N, Denmark
Clinical history
A 52-year-old man with a history of arterial hypertension was admitted because of sustained monomorphic ventricular tachycardia (Fig. A) reverted by amiodarone. Acute coronary syndrome was suspected due to inverted T-waves persisting the day after the VT arrest (Fig. B) along with elevated myocardial necrosis markers [troponin I 209, 252, and 218 ng/l on day 1 (reference <24 ng/l)]. Also, high voltage criteria for left ventricular hypertrophy (LVH) were present. Coronary angiography was normal. Echocardiography showed hypertrophic left ventricle (LV) with preserved ejection fraction (EF) and reduced strain (Fig. C). No evidence of apical sparing was evident, suggesting against cardiac amyloidosis. Viral and autoimmune serologies were normal, and near normal C-reactive protein and normal white blood cell count was disclosed.
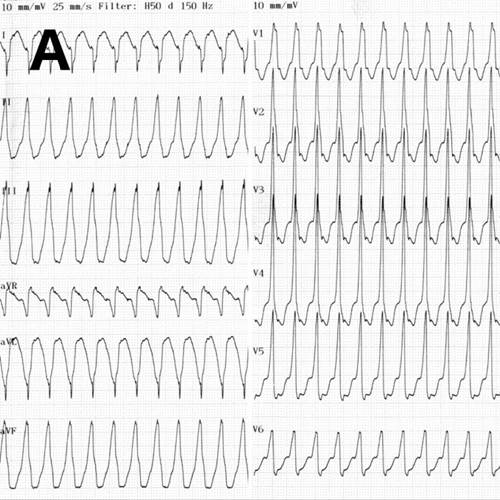
A. Ventricular tachycardia at presentation.
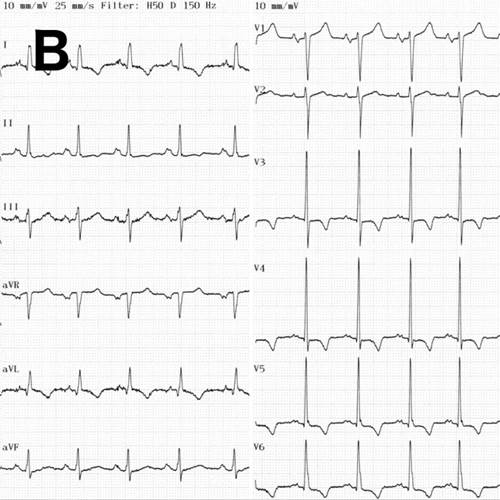
B. ECG in sinus rhythm showing inverted T-waves in leads I, II, aVL, and V3-V6.
Coronary angiography was normal. Echocardiography showed hypertrophic left ventricle (LV) with preserved ejection fraction (EF) and reduced strain (Fig. C). However, viral and autoimmune serologies were normal, and near normal C-reactive protein and normal white blood cell count was disclosed.
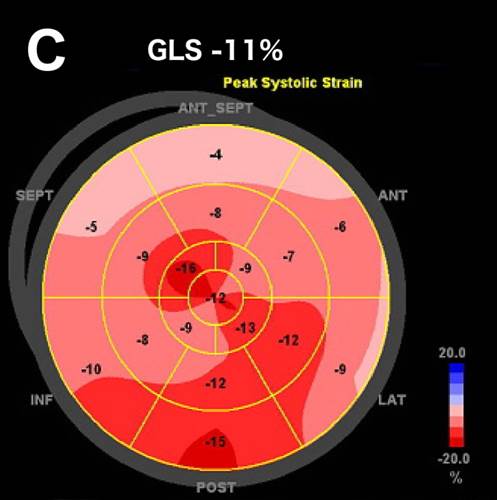
C. Bull’s eye plot of peak longitudinal strain. GLS – global longitudinal strain
Myocardial biopsy of the right ventricle showed myocardial hypertrophy with disarray and interstitial fibrosis. No inflammation, granulomas, amyloid deposits, or immunohistochemical evidence of myocarditis or sarcoidosis was found. There was no support for sarcoidosis neither on thoracic high-resolution computed tomography (CT) nor on positron emission tomography-CT with Ga-68-DOTANOC. Meanwhile, it emanated that the patient’s mother had previously been diagnosed with hypertrophic cardiomyopathy (HCM) and had died suddenly. The patient was treated with amiodarone and implantable cardioverter defibrillator. Genetic testing in 115 genes for channelopathies and cardiomyopathies revealed no disease-associated mutations.
CMR findings
Cardiac magnetic resonance (CMR) performed on day 5 confirmed LV hypertrophy with septal thickness up to 22 mm and EF 62%. The presence of mid-wall anteroseptal and inferoseptal oedema, hyperaemia and late gadolinium enhancement (LGE) was demonstrated, suggestive of possible myocarditis according to Lake Louise Criteria (Fig. D-F) [1]. Both LGE and oedema persisted on follow-up CMR on day 16.
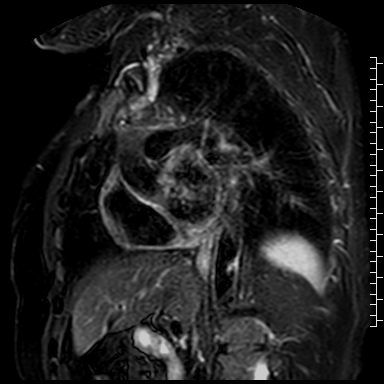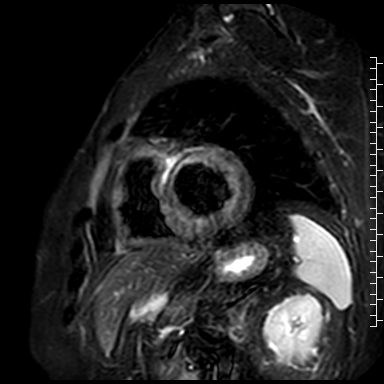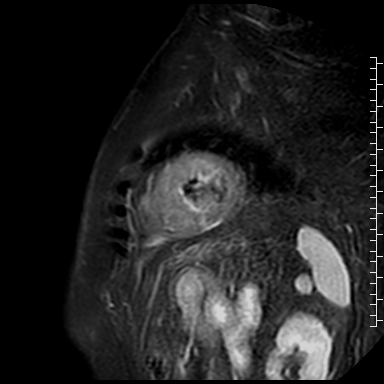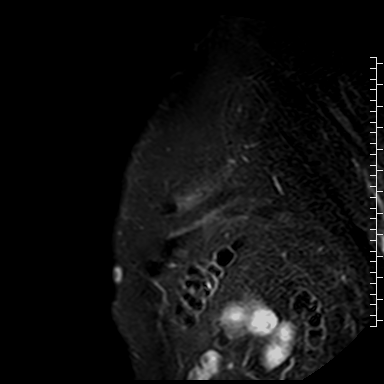
D. T2-weighted short-tau inversion recovery scan in short-axis showing myocardial oedema.
E. Early gadolinium enhancement in short-axis scan consistent with hyperaemia.
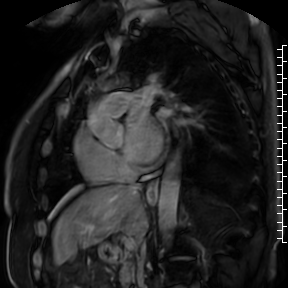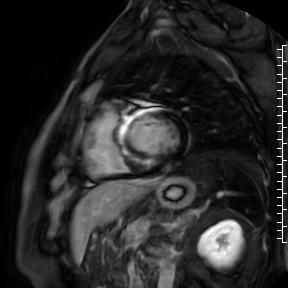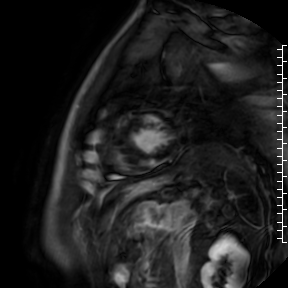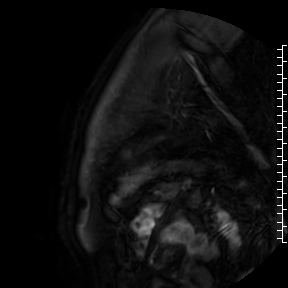
F. Late gadolinium enhancement in short-axis scan indicating myocardial scarring.
Myocardial biopsy of the right ventricle carried out on day 6 showed myocardial hypertrophy with disarray and interstitial fibrosis. No inflammation, granulomas, amyloid deposits, or immunohistochemical evidence of myocarditis or sarcoidosis was found. Due to focal inflammatory changes on CMR, thoracic high-resolution computed tomography (CT) and positron emission tomography-CT with Ga-68-DOTANOC were performed and showed no evidence of sarcoidosis. Meanwhile, it emanated that the patient’s mother had previously been diagnosed with hypertrophic cardiomyopathy (HCM) and had died suddenly. The diagnosis of HCM was also suggested by LVH and inverted T-waves on ECG. The myocardial wall thickness was above the diagnostic threshold of >=15 mm for HCM as well [2]. The patient was treated with amiodarone and implantable cardioverter defibrillator (ICD). Genetic testing in 115 genes for channelopathies and cardiomyopathies revealed no disease-associated mutations. We refrained from further CMR imaging due to the presence of ICD.
Conclusion
The present case of phenotype-positive HCM illustrates that HCM may have an acute clinical presentation showing both acute and chronic myocardial damage by CMR, mimicking inflammatory myocardial disease. However, in this particular case, myocarditis can be excluded due to normal inflammatory markers and absence of inflammation on myocardial biopsy. The final diagnosis of HCM was made based on a combination of family history, ECG, pathology, and imaging criteria.
Perspective
Patients with HCM may develop myocardial scarring detectable as LGE on CMR along with hyperaemia and oedema. The precise pathophysiological mechanism leading to myocardial necrosis in HCM is unknown but myocardial ischemia caused by microvascular dysfunction is considered one of the hallmarks of HCM [3]. On the other hand, cell damage in myocarditis is mediated by immune responses, which may be detected as LGE usually located in the subepicardial or intramural layers of myocardium, often affecting either the lateral or septal wall of the LV [1, 4]. Due to possible overlap in the imaging findings, the final diagnosis in case of oedema and LGE on CMR has to take the clinical context into account. In our opinion, further studies should address in more detail the dynamics of myocardial affection in HCM patients.
Click here to view all CMR images on CloudCMR.
References
1. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis: Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009, 53(17):1475-1487.
2. Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H: 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014, 35(39):2733-2779.
3. Maron MS: Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012, 14:13-429X-14-13.
4. Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H: Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol 2012, 59(18):1604-1615.
Case prepared by SCMR Case of the Week Associate Editor: Pranav Bhagirath





