Stephan Portner1, Paul-Martin Bansmann MD PhD1
1Department of Radiology, Krankenhaus Porz am Rhein, Cologne, Germany.
Clinical history
A 34 year old female presented with a history of loss of appetite, unintended weight loss, and heart failure. Her past history was remarkable for a diagnosis of eosinophilic polyangiitis (EGPA). She also had a prior bout of middle cerebral artery syndrome earlier in the year of uncertain etiology, although a coexisting PFO was found and thought to possibly represent a source of paradoxical embolism. A CT of the chest was performed to evaluate suspected pulmonary involvement, and demonstrated left ventricular enlargement. A transthoracic echocardiogram depicted a thrombus in the left ventricle.
A CMR exam was then performed to further evaluate this finding, assess left ventricular function, and determine possible cardiac involvement by EGPA.
CMR findings
A Siemens Skyra 3T scanner was employed to obtain imaging sequences. An LVEF of 42% was calculated with short-axis cine sequences using Simpson’s rule. Long-axis cine sequences showed a slightly dilated left ventricle with diffuse hypokinesia especially apparent at the apex in four-, three- and two-chamber views.
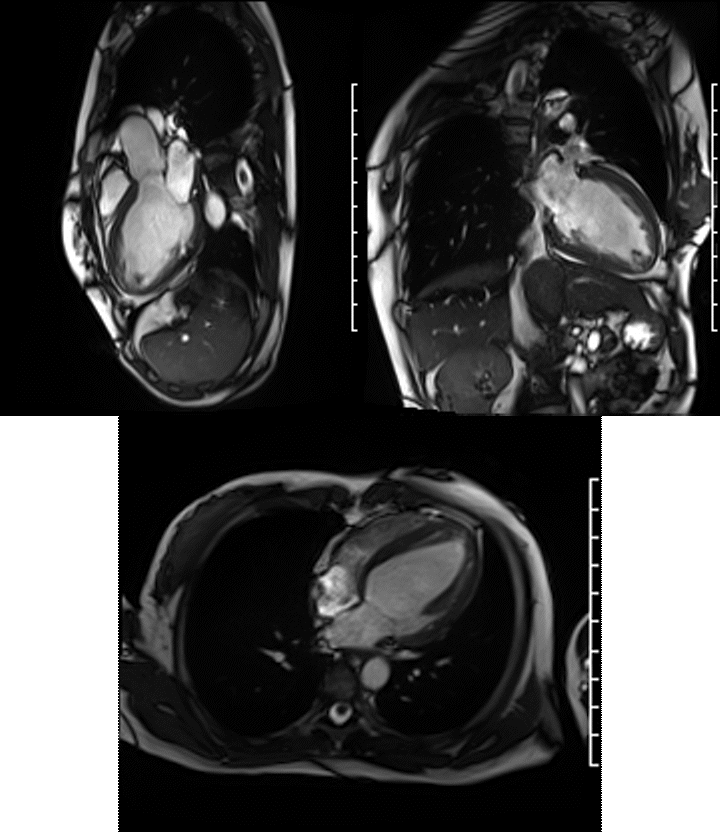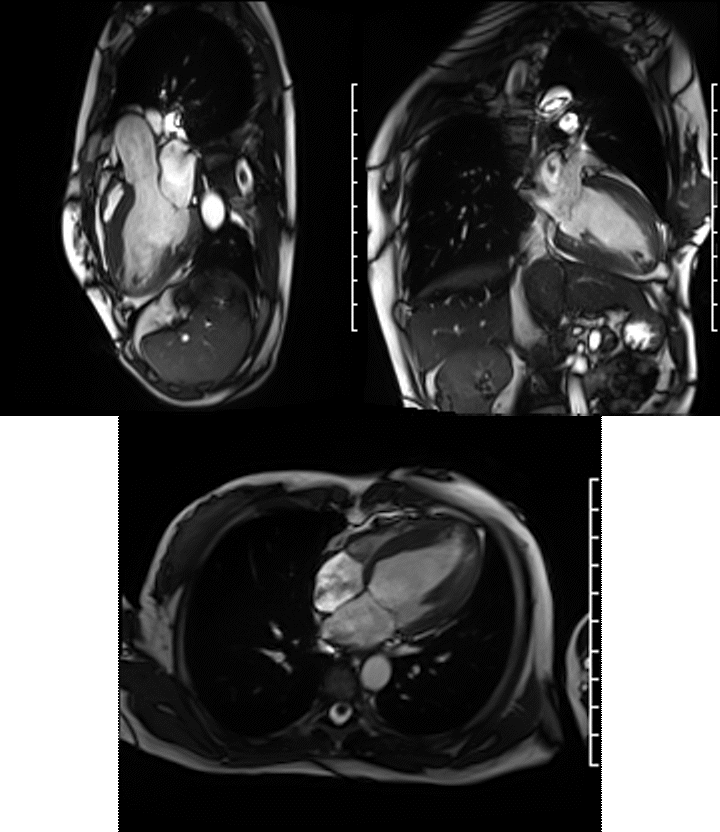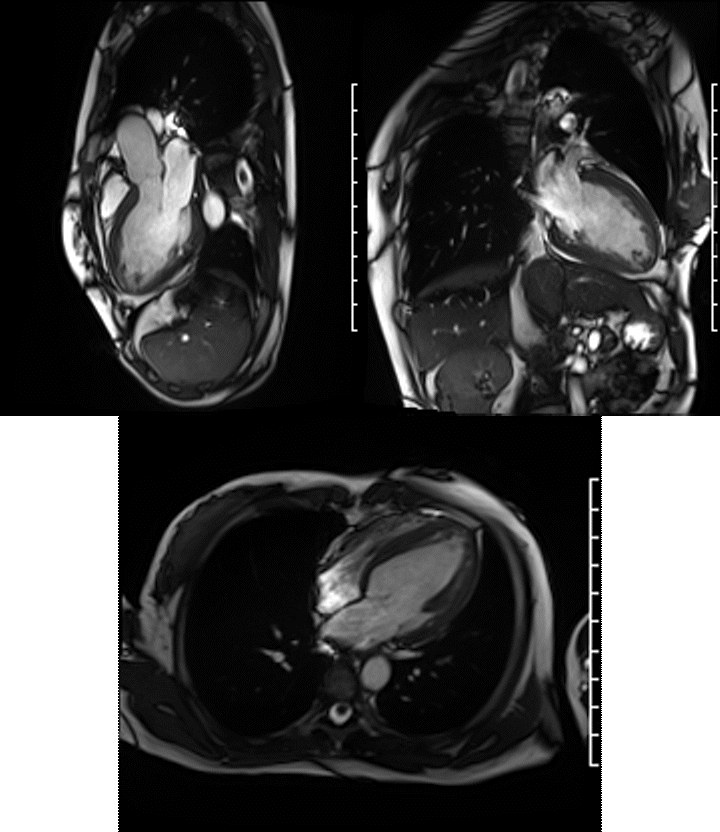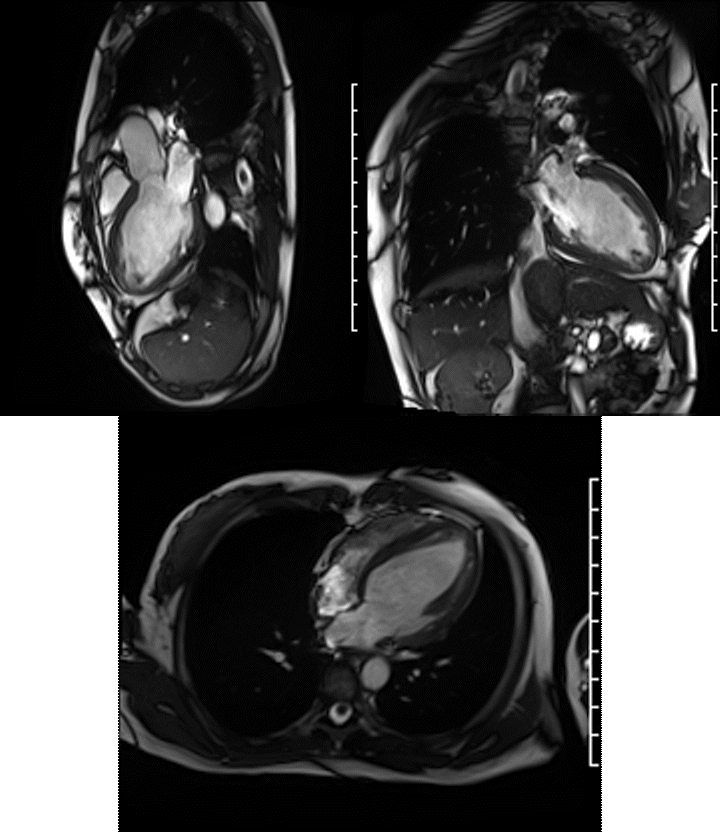
Videos: Long-axis cine imaging; 4 chamber cine (top left), 3 chamber (top right) and 2 chamber (bottom.
Suspicious findings: apical septal, basal septal and from the mid-cavity to apical lateral in short-axis T1 mapping sequences (figure below).
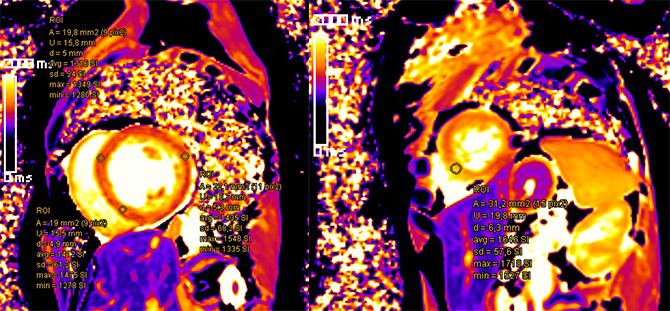
Late gadolinium enhancement showed lesions in the basal to apical septal walls and basal through apical lateral walls. In addition a small appositional thrombus was suspected in the LV apex (figures below).
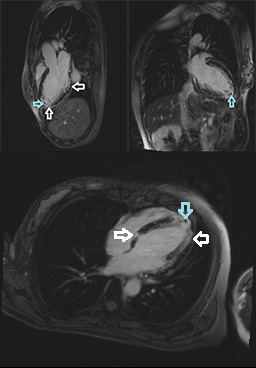
Conclusion
The diagnosis of cardiac involvement of EGPA with endomyocarditis is very likely in this case. Viral/bacterial myocarditis could also be considered. Another possibility could be endomyocardial fibrosis; however, this is unlikely due to the lack of apical thickening of the myocardium. Hypokinesia, edema, perfusion defects, and T1 and LGE abnormalities all hint at an inflammatory process such as EGPA. Transesophageal and transthoracic echocardiography confirmed a small thrombus at the apex. It is very likely that the thrombus was the etiology of the patient’s stroke. Following the diagnosis, her azathioprine was changed to rituximab and then prednisolone. Due to the presence of the thrombus, heparin was introduced and later changed to apixaban.
Perspective
Cardiac involvement is a very serious manifestation of EGPA. Endomyocarditis due to EGPA is the most severe manifestation of cardiac involvement, possibly leading to cardiac failure or fatal outcome. Severe cardiac failure may require heart transplantation (2).
Cardiac MRI can help identify impaired cardiac function and endomyocardial abnormalities (1). In a series of 22 patients with EGPA, clinical evidence (CMR findings, presence of cardiac thrombi, biopsy) of endomyocarditis was found in 13 patients (59%). Indications for CMR in asymptomatic patients are not entirely clear with studies coming to different conclusions (3;4). Other studies list CMR as an excellent tool for risk stratification and treatment individualization (5) and conclude that CMR is the most sensitive, non-invasive diagnostic technique to detect cardiac involvement (6).
This case highlights the ability of CMR to detect cardiac involvement in EGPA, especially endomyocarditis and thrombus formation, hopefully helping in utilizing adequate treatment.
Click here to view the CMR images.
References
1. 1. Neumann T1, Manger B, Schmid M, Kroegel C, Hansch A, Kaiser WA, Reinhardt D, Wolf G, Hein G, Mall G, Schett G, Zwerina J. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore). 2009 Jul;88(4):236-43.
2. Corradi D1, Maestri R, Facchetti F. Postpartum Churg-Strauss syndrome with severe cardiac involvement: description of a case and review of the literature. Clin Rheumatol. 2009 Jun;28(6):739-43.
3. Marmursztejn J1, Vignaux O, Cohen P, Guilpain P, Pagnoux C, Gouya H, Mouthon L, Legmann P, Duboc D, Guillevin L. Impact of cardiac magnetic resonance imaging for assessment of Churg-Strauss syndrome: a cross-sectional study in 20 patients. Clin Exp Rheumatol. 2009 Jan-Feb;27(1 Suppl 52):S70-6.
4. Sauvetre G1, Fares J, Caudron J, Dacher JN, Girszyn N, Daragon A, Levesque H, Marie I. Usefulness of magnetic resonance imaging in Churg-Strauss syndrome related cardiac involvement. A case series of three patients and literature review. Rev Med Interne. 2010 Sep;31(9):600-5.
5. Mavrogeni S1, Karabela G, Gialafos E, Stavropoulos E, Spiliotis G, Katsifis G, Kolovou G. Cardiac involvement in ANCA (+) and ANCA (-) Churg-Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets. 2013 Oct;12(5):322-7.
6. Miszalski-Jamka T1, Szczeklik W, Sokołowska B, Karwat K, Belzak K, Mazur W, Kereiakes DJ, Musiał J. Standard and feature tracking magnetic resonance evidence of myocardial involvement in Churg-Strauss syndrome and granulomatosis with polyangiitis (Wegener’s) in patients with normal electrocardiograms and transthoracic echocardiography. Int J Cardiovasc Imaging. 2013 Apr;29(4):843-53.
Case prepared by SCMR Case of the Week Associate Editor: Pranav Bhagirath.







