Kai Wang, MS3, Clint Walters, MD, Pascha Schafer MD, William Bates MD, Jayanth Keshavamurthy, MD, Gyanendra K. Sharma, MD, Darko Pucar, MD.
Medical College of Georgia, Augusta, GA, United States
Introduction
Early diagnosis of aortitis is crucial for proper management of the disease and prevention of future sequelae. Clinical presentation of aortitis is often nonspecific and patients with early stages of aortitis are often asymptomatic. Traditionally, the diagnosis of aortitis is invasive often requiring arterial biopsy. However, advanced imaging can be an invaluable tool to assist clinicians in the evaluation of patients for possible aortitis with high specificity, and sensitivity [4]. In this case report, we present a patient with atypical presentation of aortitis, and describe how different imaging modalities can be used to assist in the diagnosis of aortitis.
Presentation
A 43-year-old female with a history of asthma and uterine fibroids presented with shortness of breath. She reported chest tightness and was awakened from sleep with chest pain that radiated down her left arm. She had no prior history of cardiac diseases. There was recent history of fever, and productive cough.
Contrast enhanced chest computed tomography (Figure 1) showed a large circumferential pericardial effusion measuring 2.3 cm. Since she demonstrated a viral prodrome 3 days prior, viral pericarditis was the initial working diagnosis. A follow up echocardiogram 1 week later showed a decrease in the size of the pericardial effusion without steroid therapy. The patient was asymptomatic 1 week after being discharged from the hospital, and an echocardiogram demonstrated complete resolution of the prior pericardial effusion.
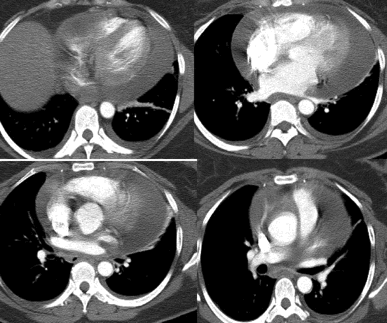
Figure 1
The patient came back 1 year later for progressive chest pain, dyspnea, lower extremity swelling, and decreased exercise tolerance. A Trans Thoracic Echocardiogram (TTE) showed preserved ejection fraction, mild to moderate aortic insufficiency, mild mitral regurgitation, mild tricuspid regurgitation and no effusion, with mild thickening of pericardium. The patient’s symptoms were out of proportion for simple valvular dysfunction. Nuclear medicine myocardial perfusion imaging was performed to rule out ischemic cardiac disease, and the result was normal. A gated cardiac CT was ordered to evaluate for constrictive pericarditis.
Cardiac Gated CTA (Figure 3) performed one year after the initial presentation showed an ascending aorta diameter of 42 mm which was an increase from 31 mm one year prior (Figure 2). Furthermore, Cardiac gated CT also showed irregular thickening of the wall of the ascending aorta with increased mural attenuation of the aortic wall on non-contrasted images.
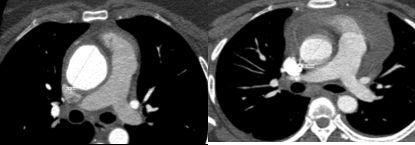
Figure 2 & Figure 3
T2 weighted MRI was performed with a 3-T MRI system (Philips). Images obtained from the study showed increased T2 signal in the tunica media of the ascending aorta and mildly thickened aorta wall up to 5mm (Figure 4). Contrast enhanced MRI showed patchy enhancement of the ascending aorta wall. (Figure 5). MRI findings were highly suggestive of inflammatory aortitis. Neither cardiac gated CT nor MRI showed evidence of pericardial thickening or findings of constrictive pericarditis.
MRI and Cardiac Gated CT results indicated the presence of inflammatory changes of the aorta. Since our patient’s clinical presentation was neither typical of Takayasu or Giant Cell Arteritis, 18F-FDG PET/CT was ordered to rule out IgG4-related sclerosing disease [7].
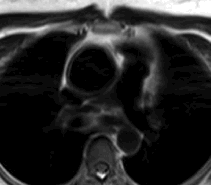
Figure 4
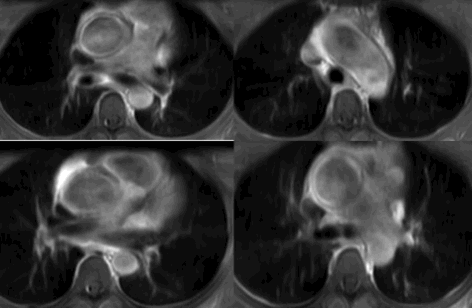
Figure 5
18F-FDG PET/CT showed ascending aorta dilation of 4.5 cm and localized increased activity in the right lateral wall of the ascending aorta with standardized uptake value (SUV) of 4.8, within the region of wall thickening. (Figure 6).
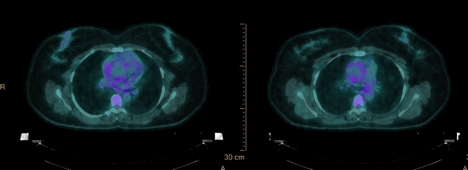
Figure 6
Further laboratory evaluation (Figure 7) showed negative viral titers and serology. The patient’s ESR (34 mm/hr), CRP (2.182 mg/L) were mildly elevated, and IgG 4 (19.5 mg/dL) level was normal. The patient was started on 20mg oral prednisone and her symptoms improved. She could now complete 20 minutes of strenuous exercise.
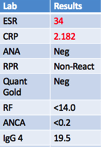
Figure 7
4 month follow up 18F-FDG PET/CT showed markedly decreased right lateral aorta wall metabolic activity with SUV of 2.5, and stable ascending aortic root dilation of 4.6 cm.(Figure 8).
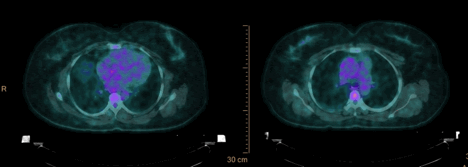
Figure 8
Our patient’s final diagnosis was idiopathic aortitis based on laboratory results and imaging findings. Later she was switched to azathioprine therapy from oral prednisone. By prompt diagnosis and treatment, we hope to prevent the need for her to undergo aortic aneurysm surgery. Three years later, she is doing well, her aneurysm size has stabilized, and she attends cross training classes regularly.
Discussion
Pericardial effusion is a common finding in clinical practice. However, it can be challenging for the clinician to establish an etiologic diagnosis in some cases, since many conditions can cause pericardial effusion.
The pericardium surrounds the heart extending superiorly to cover the main pulmonary artery, ascending aorta, and superior vena cava. Therefore, inflammation of the ascending aorta can result in exudative effusion in the pericardial sac. However, aortitis is an uncommon cause of pericardial effusion. Therefore, our patient’s aortitis diagnosis was delayed due to the atypical presentation of her disease.
Aortitis can be caused by different etiologies. The most common cause of aortitis is large vessel vasculitis such as Giant Cell Arteritis (GCA), Takayasu Arteritis (TA), and idiopathic aortitis. All three types of arteritis have similar histological findings. However, GCA commonly occurs in patient older than 50 years old with a peak incidence of 80 years of age [1]. TA usually occurs in female less than 40 years old with Asian females most commonly affected. Idiopathic aortitis (IA) most commonly occur in smokers and has a younger age of onset.
The Cleveland Clinic performed a retrograde study of 1,204 aortic surgical specimens. This study found that 4.3% of patients could be classified as having IA [5]. IA patients generally do not have underlying systemic diseases nor rheumatologic symptoms. The majority of IA patients present with aneurysm, and aortitis is most commonly present in the thoracic aorta. Patients with IA often require regular follow up because of the propensity for aneurysm formation [6].
Large vessel vasculitis differs from other vasculitis as it is mediated by CD4+ T cells, antigen presenting cells, and macrophages. Histologically transmural inflammation is a common shared feature. This inflammation eventually causes arterial stenosis, vascular wall thickening, thrombosis and aneurysms. Definitive diagnosis of aortitis often requires biopsy to demonstrate inflammatory cellular infiltrate of the vascular wall.
Aortitis can present with a variety of symptoms and often patients with early stages are asymptomatic. The unique presentations of aortitis and invasive nature of arterial biopsy can cause delayed diagnosis of large vessel arteritis. Recent advances in non-invasive imaging may be an invaluable tool to aid in the early diagnosis of large vessel arteritis.
MRI enables clinicians to assess conditions of the vessel wall to evaluate for wall thickness, luminal stenosis, and vessel wall edema. The presence of these findings are highly suggestive of active arteritis. PET can effectively detect increased metabolic activities within vessel walls [2]. PET imaging often can be used to determine the extent of vessel wall involvement, and some studies suggest PET imaging can also be used to gauge patient’s response to treatment [3].
Conclusion
The early diagnosis of large vessel arteritis can be challenging, and clinician need to have high index of suspicion when evaluating patients. Different imaging modalities are a great aid for the early diagnosis of large vessel arteritis.
Prompt recognition and appropriate immunosuppressive therapies can drastically improve acute symptoms. Patients with large vessel arteritis require frequent follow ups and relapse is very common.
Click here to view the CMR images.
Case prepared by Associate Editor: Madhusudan Ganigara
References
1. Gulati A, Bagga A. Large vessel vasculitides. Pediatr Nephrol. 2010;25:1037–48.







