Elsio Negron-Rubio MD, Aamisha Gupta MD, Donald Cifelli, Gyanendra K Sharma MD, Kenneth A. Murdison MD, Jayanth H Keshavamurthy MD, Augusta University, Department of Pediatrics, Division of Pediatric Cardiology and Department of Radiology, Augusta, GA.
History:
The patient is a 3-week-old male with a history of factor VII deficiency presented with new onset right shoulder swelling. He was brought to the ED after his mother noticed that his shirt was fitting differently and that he was favoring use of his left arm. The mother further reported a hard lump on his left ribcage. She stated that it “just appeared”. She also reported labored breathing, with substernal retractions and tachypnea. On monitor the patient’s oxygenation remained above 99%. These episodes were not related to feeding, and the patient was calm when sleeping. CXR on admission was within normal limits. ECG showed sinus tachycardia.
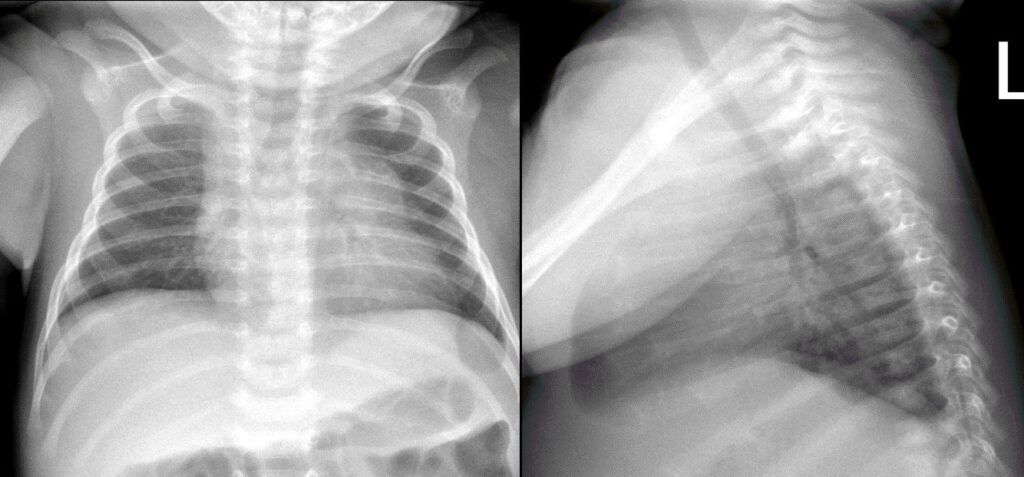
Figure 1: Frontal and lateral chest radiograph
Pediatric Cardiology was then consulted due to continued swelling and a concern for SVC syndrome. As per cardiology recommendations an echocardiogram was obtained. The echocardiogram demonstrated a moderate-large sized irregular non-homogenous mediastinal mass, localized to the space cranial to and rightward of the heart, adjacent to but distinct from the thymus. This mass was described as having several echogenic areas, as well as multiple hypoechoic structures resembling vessels, but without demonstrable flow on color Doppler interrogation. The heart was otherwise structurally normal. The SVC was well seen and had a normal flow profile. The RV was mildly dilated and demonstrated normal systolic function. The LV showed normal cavity size and systolic function (LV shortening fraction= 36% ). Considering these results, SVC syndrome was ruled out with confidence.
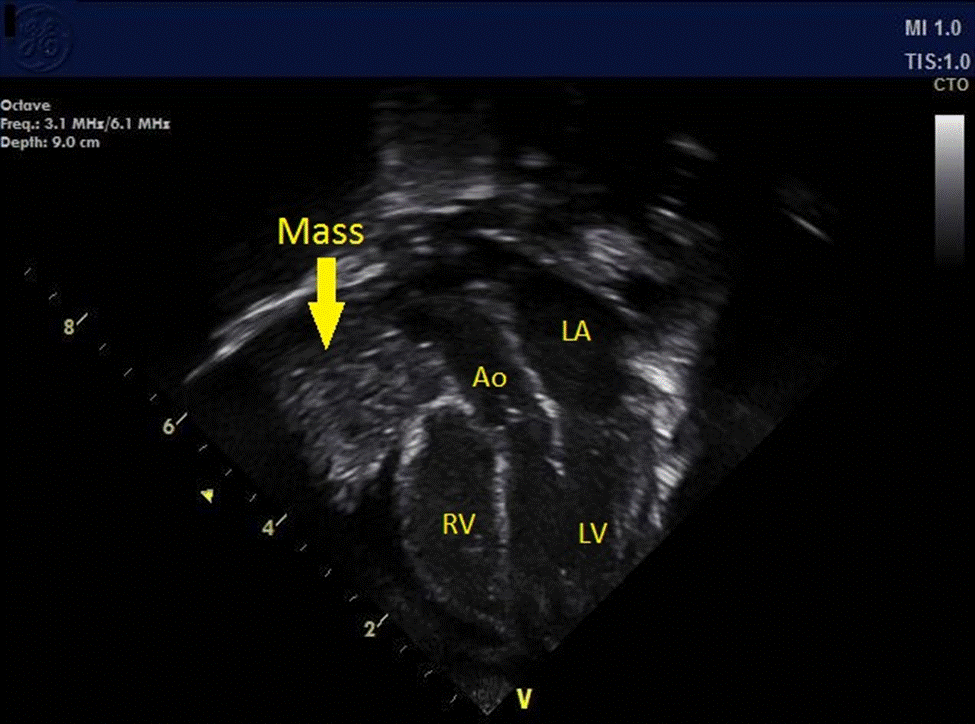
Image 2: Still frame of the same 4-chamber transthoracic echocardiographic image of heart and heterogeneous mass (arrow) demonstrated to the right of the right atrium and right ventricle. Aorta (Ao). Left Atrium (LA). Left Ventricle (LV). Right Ventricle (RV).
CT of the chest without contrast was performed initially as a first choice for further characterization as this did not require sedation unlike cardiac MRI which is done under general anesthesia. CT showed a large soft tissue mass in the anterior mediastinum with central hypodensity. Given the heterogeneity and configuration of this mass, the finding was reported as concerning for a neoplastic process in the anterior mediastinum rather than normal thymus. The mass would have been better evaluated with contrast, but unfortunately, due to technical problems with contrast infusion, contrast enhanced images were not adequately obtained.
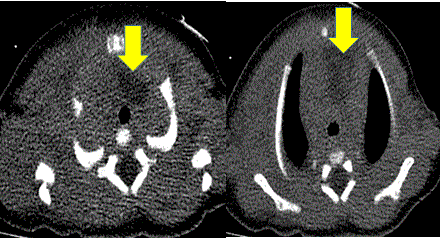
Figure 3: Non-contrast CT chest images: There is a low density area to the left of trachea extending into the anterior mediastinum and anterior to the heart. There is no tracheal deviation.
CMR Findings:
Following the inconclusive CT scan, the patient was sent for emergent Cardiac MRI to further evaluate the mediastinal mass. The MRI was obtained on a 3T Philips Insignia. Although pediatric anesthesia was consulted for this case it was determined that the potential respiratory decompensation due to an anterior mass along with factor VII deficiency presented a high risk for sedation and intubation. Therefore, the MRI was obtained free breathing without sedation. The patient was given sucrose and was bottle fed immediately prior to the MRI scan to assist in reducing motion artifact and allow for acquisitions of diagnostic quality.
MRI protocol included axial, coronal, and sagittal T1 (TR 352, TE 25) and T2 weighted images, including single shot Turbo Spin Echo (TSE; TR 1500, TE 160) images with and without fat saturation. Cine images were acquired in the axial plane to include the mediastinal mass, great vessels, and heart. Perfusion imaging in an oblique plane was acquired to maximize the coverage of the mass.
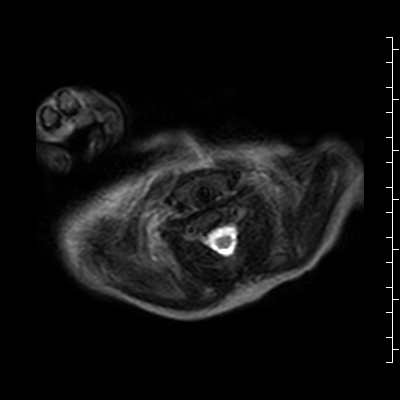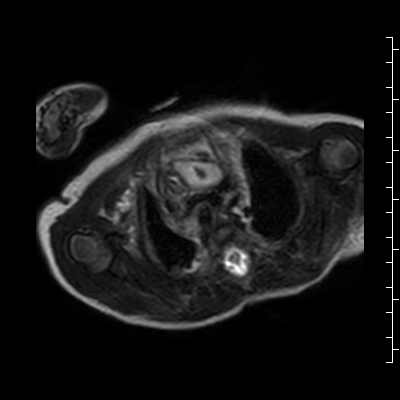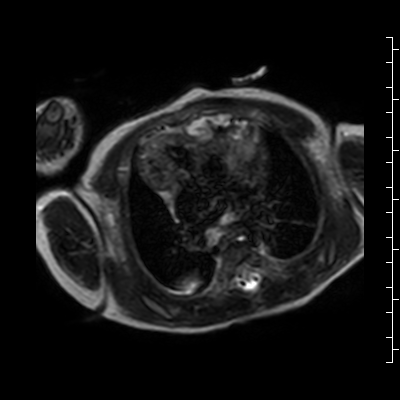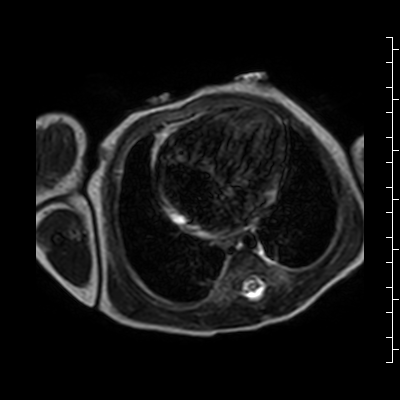
Video 2: Single shot turbo spin echo images in an axial stack through the chest demonstrating the anterior mass and its relationship to the thymus and great arteries
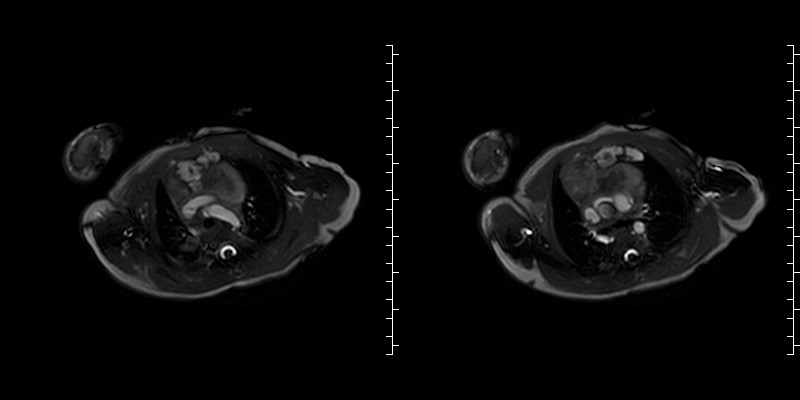
Figure 4: T2 weighted images demonstrate a fluid filled structure displacing the thymus to the right of midline.
T1 weighted images were then performed which demonstrated a large (1.1 x 3.3 x 5.0 cm in the AP, transverse and craniocaudal dimensions) lobulated, thinly septated lesion with high signal on both T1 (Fig 5) and T1 fat suppressed images (Fig 6).
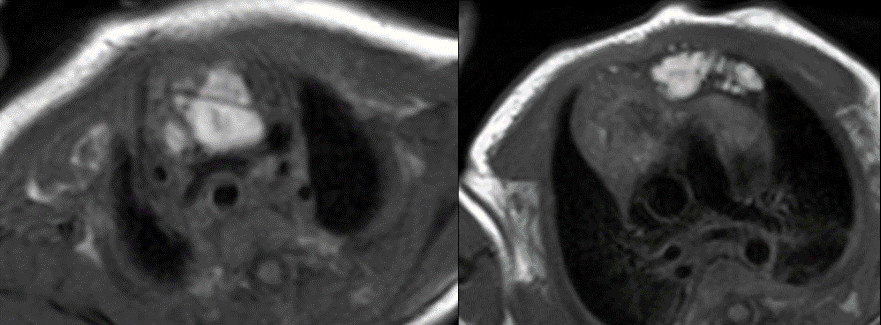
Figure 5: T1 without fat suppression. There are linear hyperintense lesions extending from the neck into the anterior mediastinum and displacing the thymus to the right.
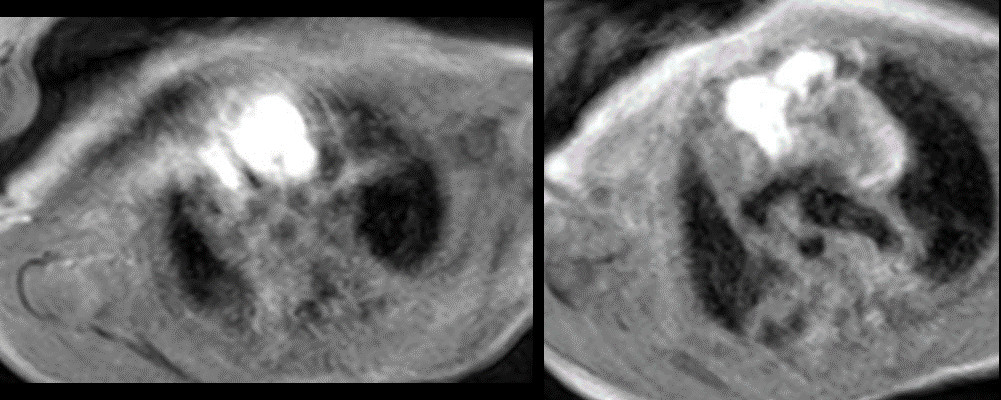
Figure 6: T1 with fat suppression. There are linear hyperintense lesions extending from the neck into the anterior mediastinum and displacing the thymus to the right. There was no fat suppression of the lesion.
On T2 weighted images, there was high signal identified within this lesion with interspersed areas of low signal intensity.
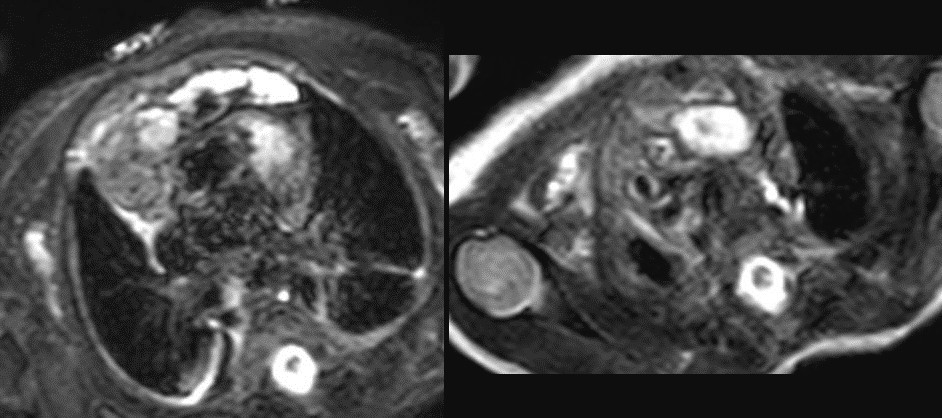
Figure 7: T2 weighted images. Lateral to this structure, on the right there is a T1 and T2 low signal intensity lesion that is isointense to muscle favored to represent normal thymic tissue.
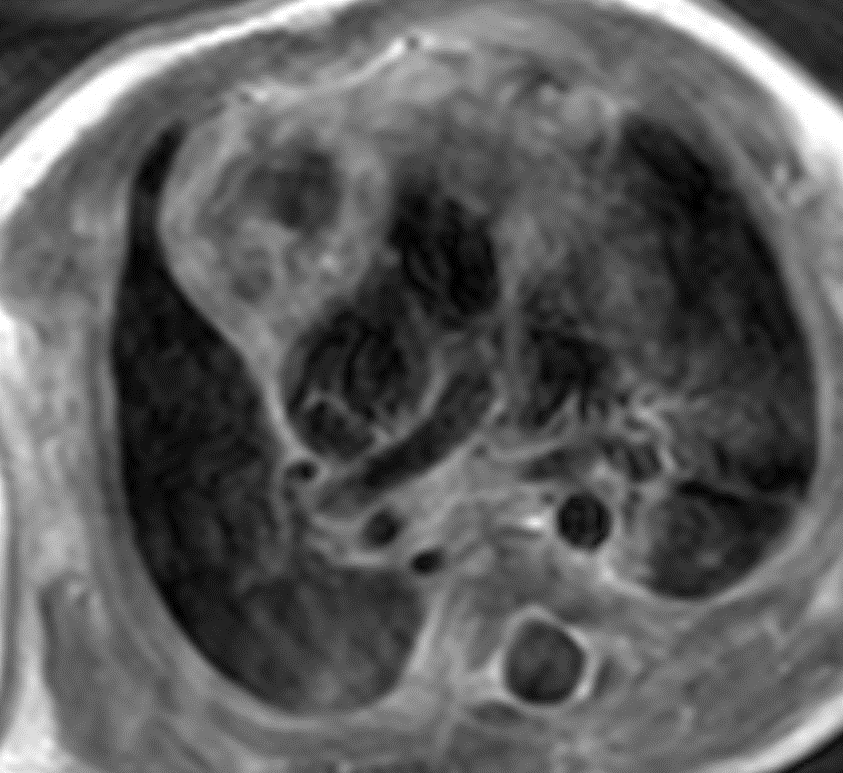
Figure 8: Post contrast T1 weighted turbo spin echo image. The thymus is displaced to the right side of the heart by the mass. The periphery of the thymus and capsule enhance post contrast. The mass itself showed no increased perfusion on dedicated perfusion imaging and no internal enhancement on post contrast imaging.
Conclusions:
Taking into consideration all characteristics, a lymphatic malformation extending from the left side of the neck to the anterior mediastinum, with somewhat atypical T1 behavior secondary to increased internal proteinaceous components, was determined to be the most likely diagnosis. Although limited by lack of sedation, MRI was still able to characterize the nature of this mass using T1 and T2 imaging with and without fat saturation. Contrast injection was able to further differentiate the lesion between normal thymic tissue and other vascular malformations.
Taking steps prior to the MRI such as use of sucrose, bottle feeding immediately prior to the scan, and tight papoosing with warm blankets assisted in allowing for acquisition of images of diagnostic value. Correlating the patient history, the physical exam, and findings from different imaging modalities, a final accurate diagnosis was possible. Now with a definitive diagnosis, discussions about what is the best management approach for this patient can be undertaken. In our case, biopsy of this lesion was not considered due to high risk of procedural bleeding given patients history of factor VII deficiency.
Perspectives:
Lymphatic Malformations (LMs), previously called cavernous lymphangioma, cystic hygroma, cystic lymphangioma and lymphangioma circumscriptum, are benign mass lesions consisting of chyle-filled cysts lined with endothelium. They are the second most common type of vascular malformation after venous malformations and result from sequestered lymphatic sacs that fail to communicate with peripheral draining channels. Although some present at birth, most are discovered in the first two years of life. LMs occur most commonly in the head and neck (48%), in the trunk and extremities (42%) and in the intrathoracic or abdominal compartment (10%). They usually present as noncompressible, rubbery soft tissue lesions, which can be solitary or multifocal and usually grow slowly, but can rapidly expand due to hemorrhage or infection. Regardless of size, LMs can potentially cause functional impairment and/or disfigurement of nearby structures.
There are two major subtypes of LMs assessed radiographically: macrocystic and microcystic. Ultrasound is the initial modality in evaluating these vascular malformations. In the case of LMs and venous malformations (VMs), ultrasound shows little or no flow on color Doppler images (low flow lesions). In order to further characterize and differentiate these low flow lesions, MRI is the usual next step.
Macrocystic LMs are characterized by a single or multiple fluid filled cysts, usually more than 2cm in diameter. These are usually easily differentiated from VMs by their predominantly cavitary, lobulated and septated appearance that is intermediate/hyperintense to hypointense on T1-weighted images, depending on the amount of proteinaceous material, and hypertense on T2-weighted images and STIR images. In contrast to VMs, macrocystic lesions will not enhance except in possible rings and arcs within the cyst wall or septae. (1) On the other hand, microcystic LMs are characterized by cysts/vesicles smaller than 2 mm and many times are imperceptible. Microcystic lesions are differentiated from macrocystic varieties by their relative absence of dominant cystic spaces and can be differentiated from VMs by their usual lack of contrast enhancement. Microcystic lesions are also usually hypointese on T1-weighted images and hyperintense in T2-weighted images.
When considering treatment for these lesions, current available options are sclerotherapy, percutaneous drainage, surgery, and laser therapy and radiofrequency ablation. Sclerotherapy in particular has shown to be very effective in macrocystic lesions (2).
Click here to view all CMR images for the case on CloudCMR
References:
1. Flors L, Leiva-Salinas C, Maged IM et-al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011;31 (5): 1321-40.
2. Legiehn GM, Heran MKS. A Step-by-Step Practical Approach to Imaging Diagnosis and Interventional Radiologic Therapy in Vascular Malformations.Seminars in Interventional Radiology. 2010;27(2):209-231







