Arun Dahiya1,2 MBBS FRACP, Charles Chao1 MBBS FRACP, John Younger1 MBBS FRACP, Chris Hammet1 MBBS FRACP
1Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia.
2GriffithUniversity School of Medicine, Gold Coast, Queensland, Australia.
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical History
A 19-year-old woman presented with a 2-week history of painful atraumatic left ankle swelling associated with painful plantar-flexion and petechial rash on the left foot. Six weeks prior to presentation, she experienced flu-like illness with headache, fevers and myalgias/athralgias. On examination, there was a non-blanching petechial rash over the dorsum of the left foot with associated edema and warmth but no erythema. Peripheral pulses were normally palpable with normal capillary refill. Neurological exam of the foot was normal except for 4/5 power on plantar flexion due to pain. Ultrasound of the leg showed a thrombus in the lateral tarsal artery raising a possibility of an embolus.
Her background was remarkable for mild childhood asthma that was associated with with viral infections and exercise, and allergic rhinitis. She had no prior hospitalizations. Chest X-ray was unremarkable. ECG showed T-wave inversion in most precordial leads (V2-V6) and some inferior leads (III and aVF) (image 1).
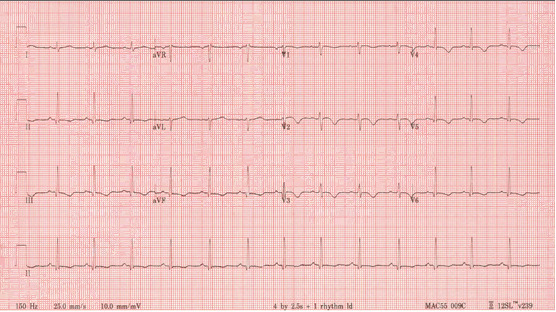
Image 1: 12 lead ECG showing sinus rhythm with T-wave inversion in most precordial leads (V2-V6) and some inferior limb leads (III and aVF).
Routine blood work was unremarkable except for eosinophilia which was attributable to asthma. Inflammatory markers (ESR 41mm/hr and CRP 22) were mildly elevated. Standard troponin was negative. Trans-thoracic echocardiogram performed to evaluate for a possible cardiac embolic source was unremarkable except for mildly increased left ventricular (LV) apical wall thickness (Video 1). Given the abnormal ECG and increased LV apical wall thickness, cardiac MRI (CMR) was requested to rule out apical hypertrophic cardiomyopathy.
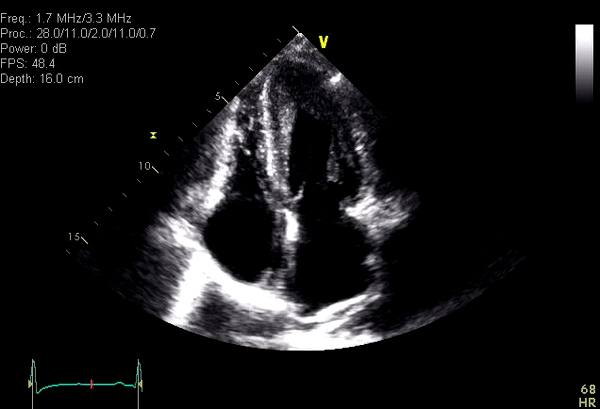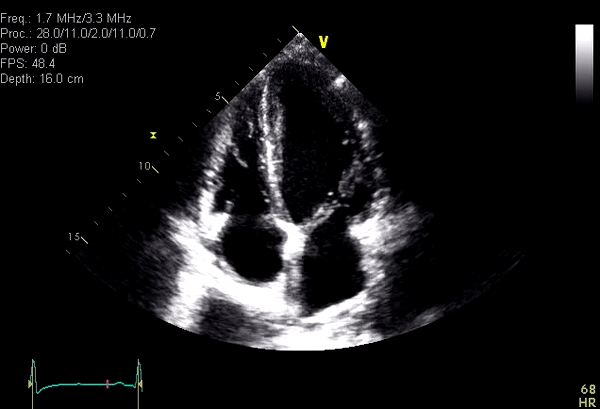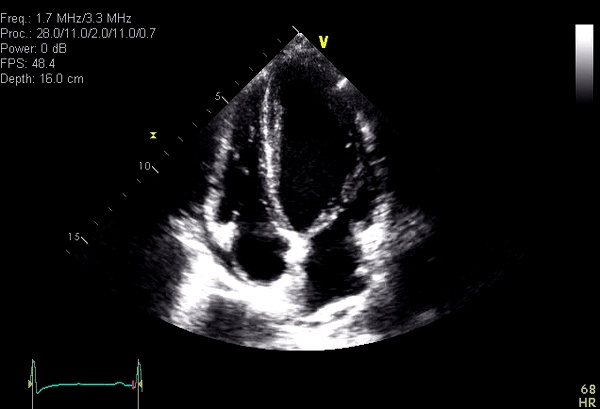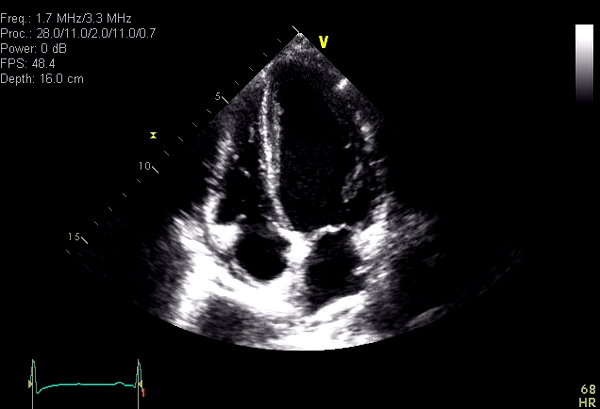
Video 1: Apical 4 chamber obtained from a technically difficult Transthoracic echocardiographic study showing systolic obliteration of the LV apex. Although LV apical diastolic thickness was only minimally increased, apical HCM remained a concern because of suspected LV apical foreshortening.
CMR Findings
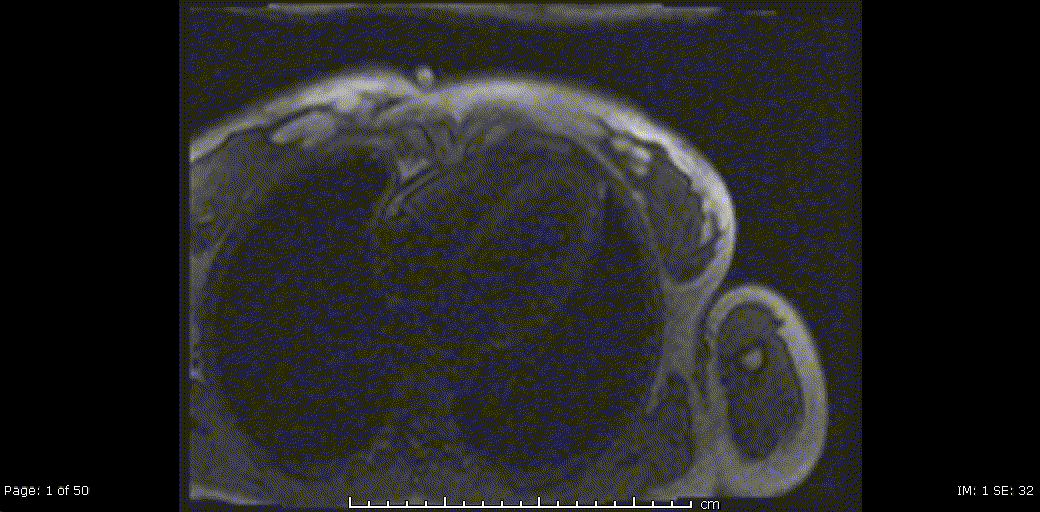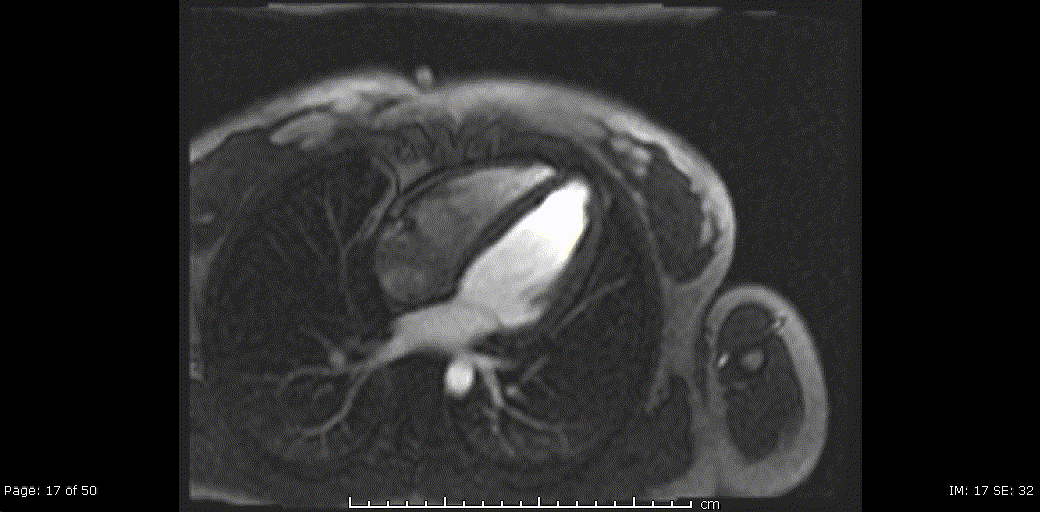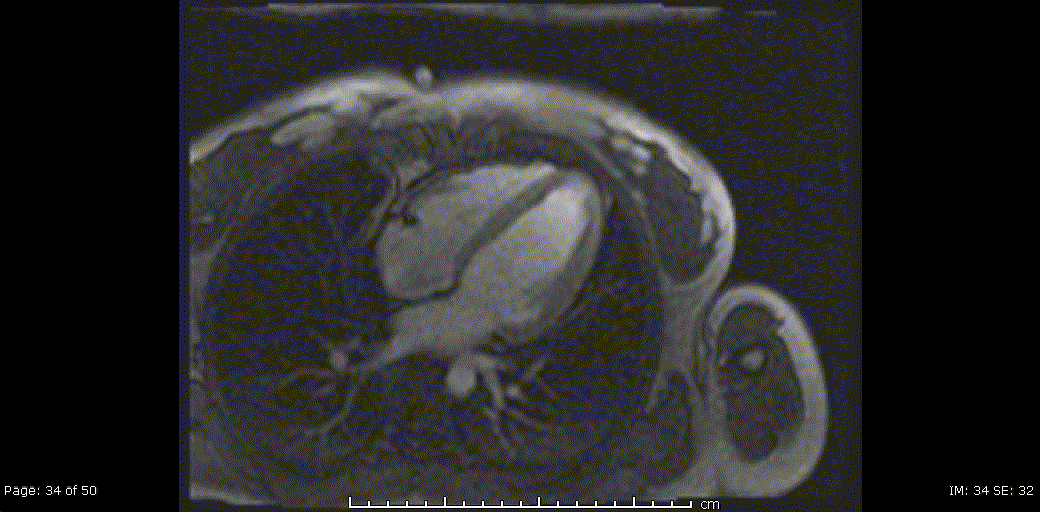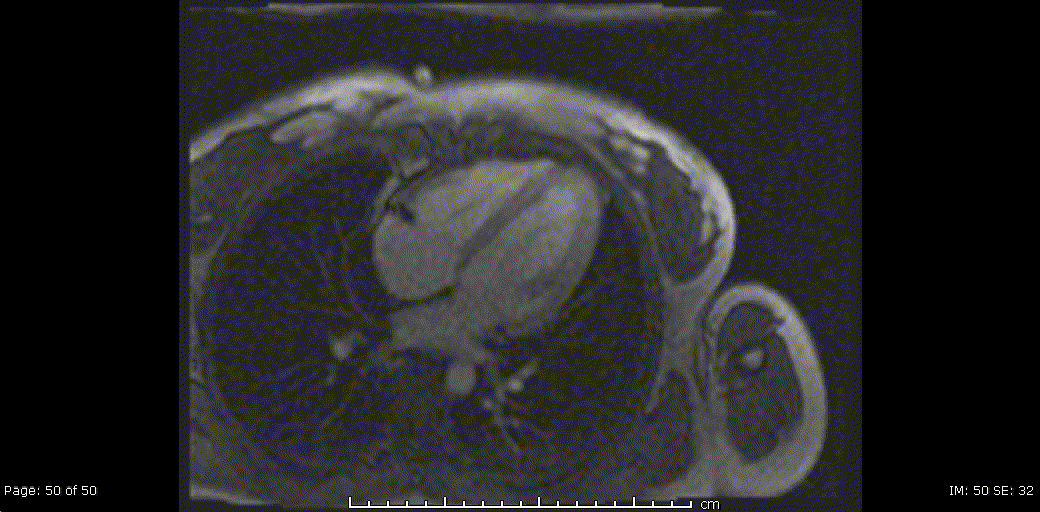
Cardiac MRI showed normal biventricular size and systolic function with only minimal increase in the LV apical wall thickness (Video 2,3,4).
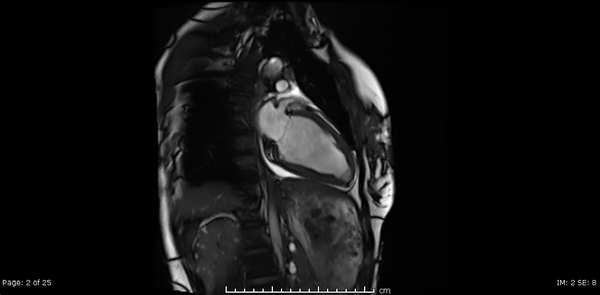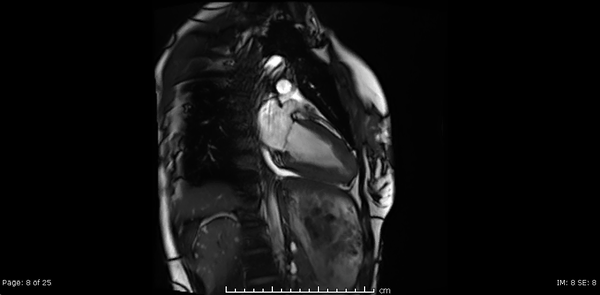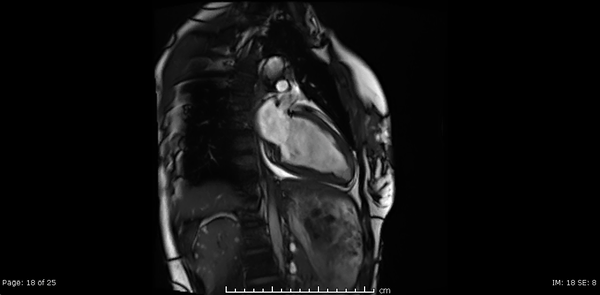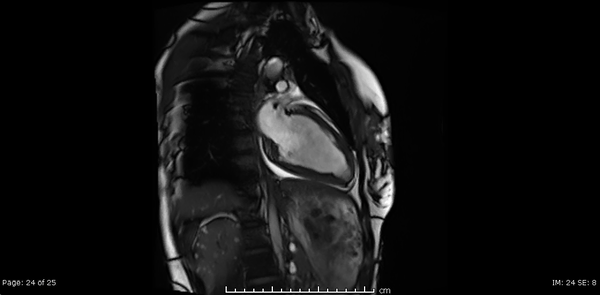
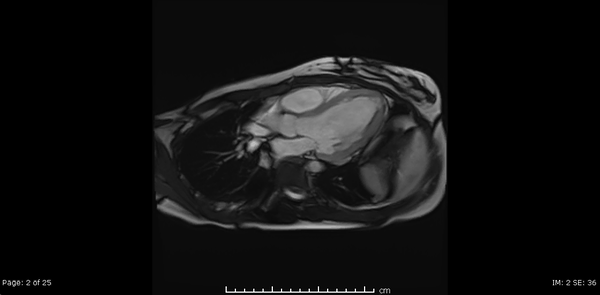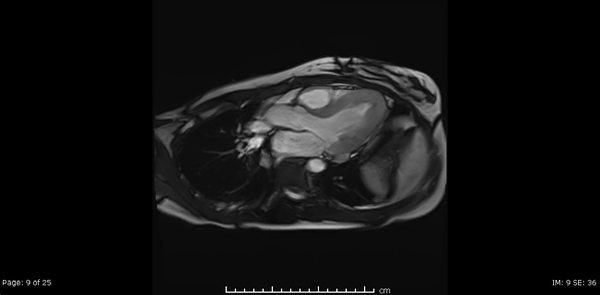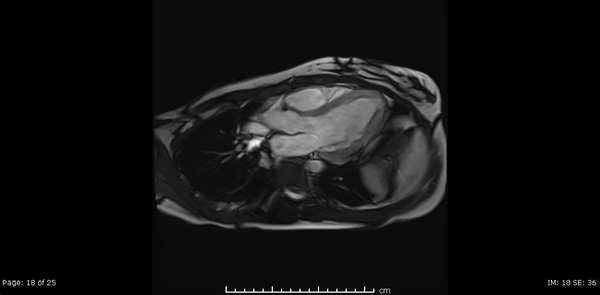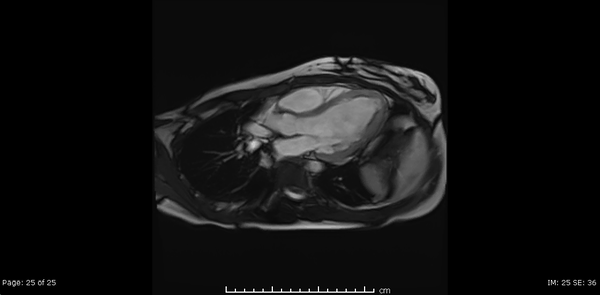
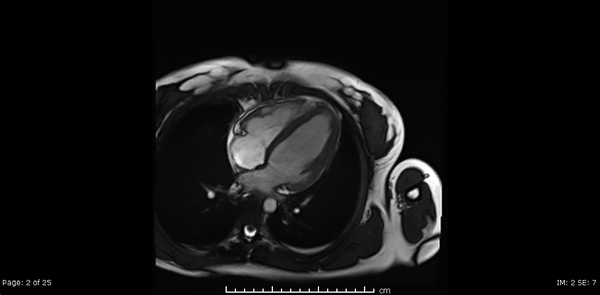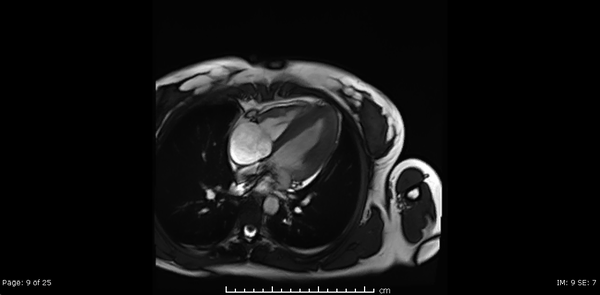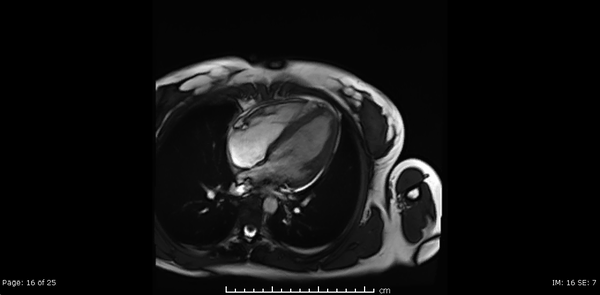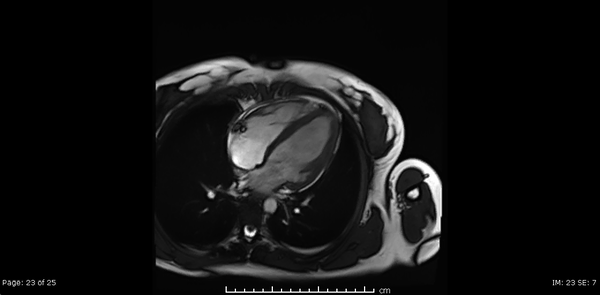
Video 2, 3,4: SSFP cine sequences of the 2-, 3- and 4-chamber views respectively.
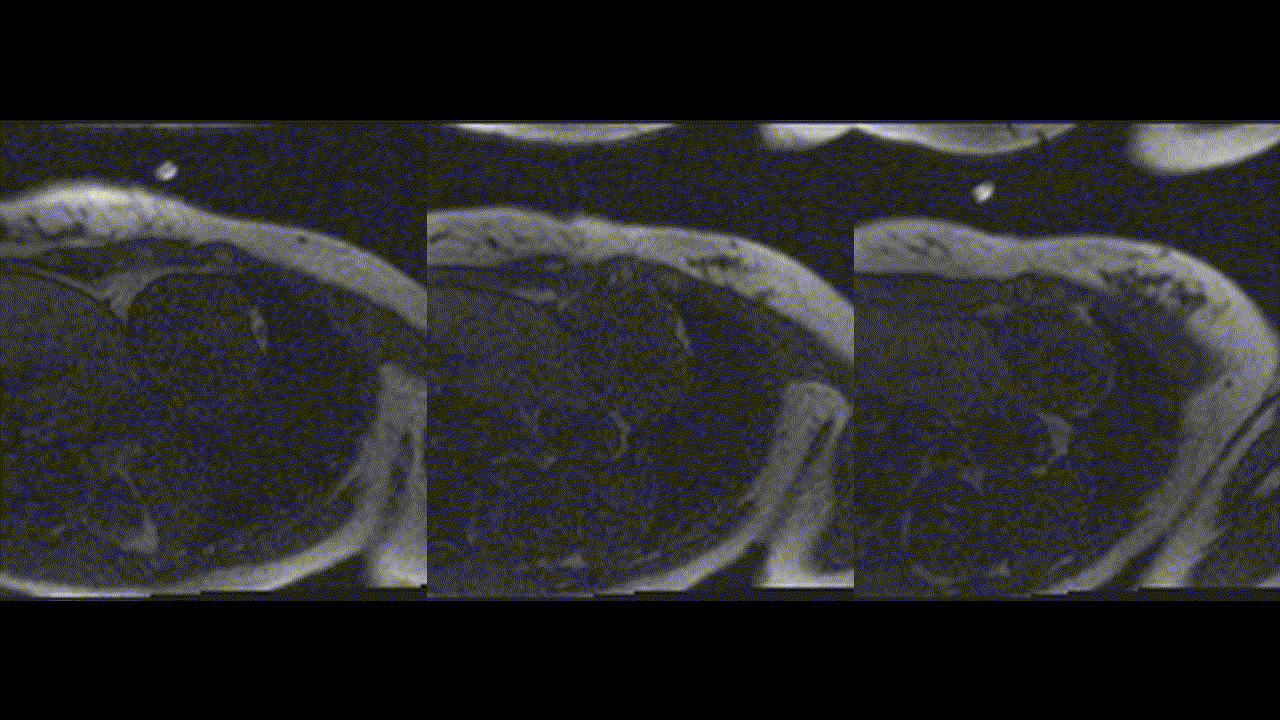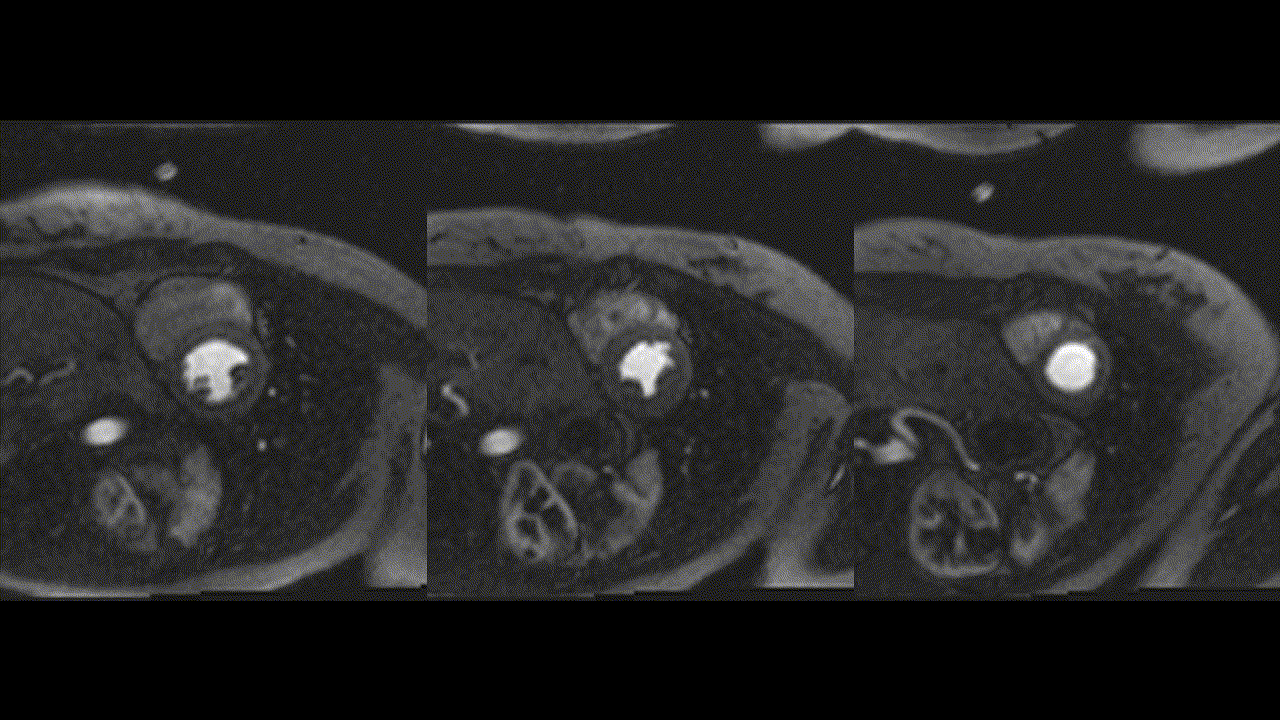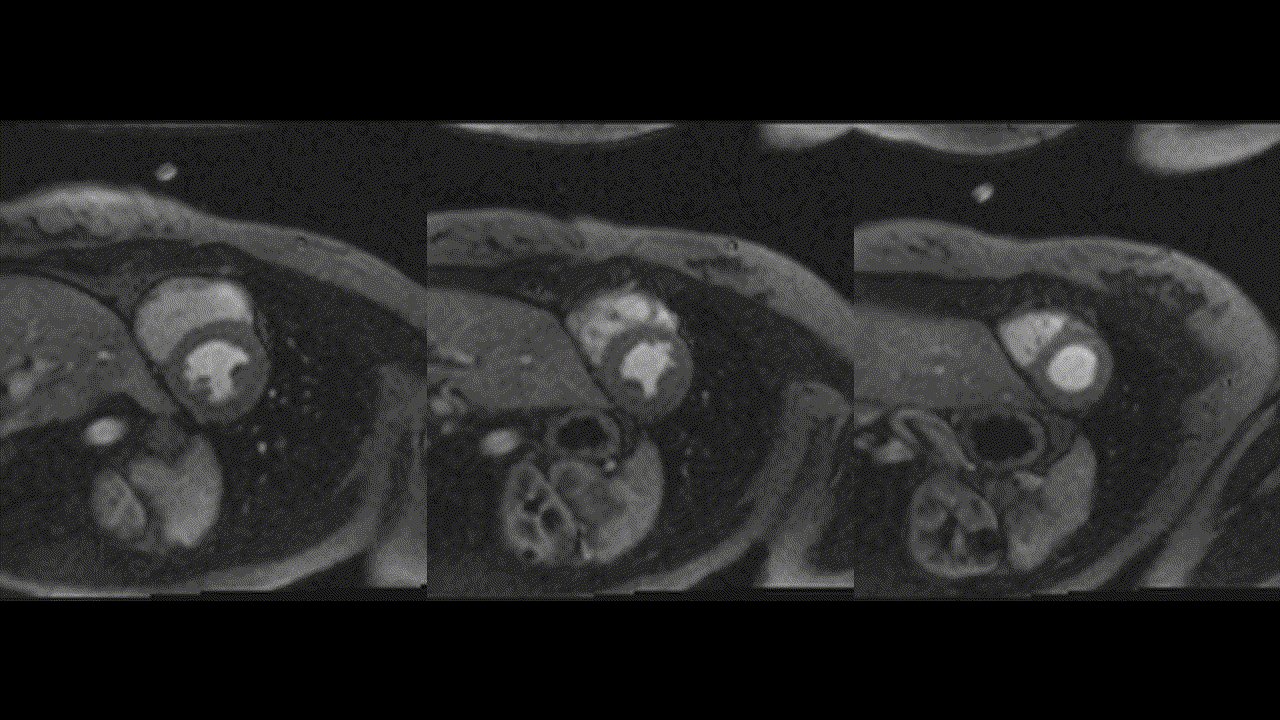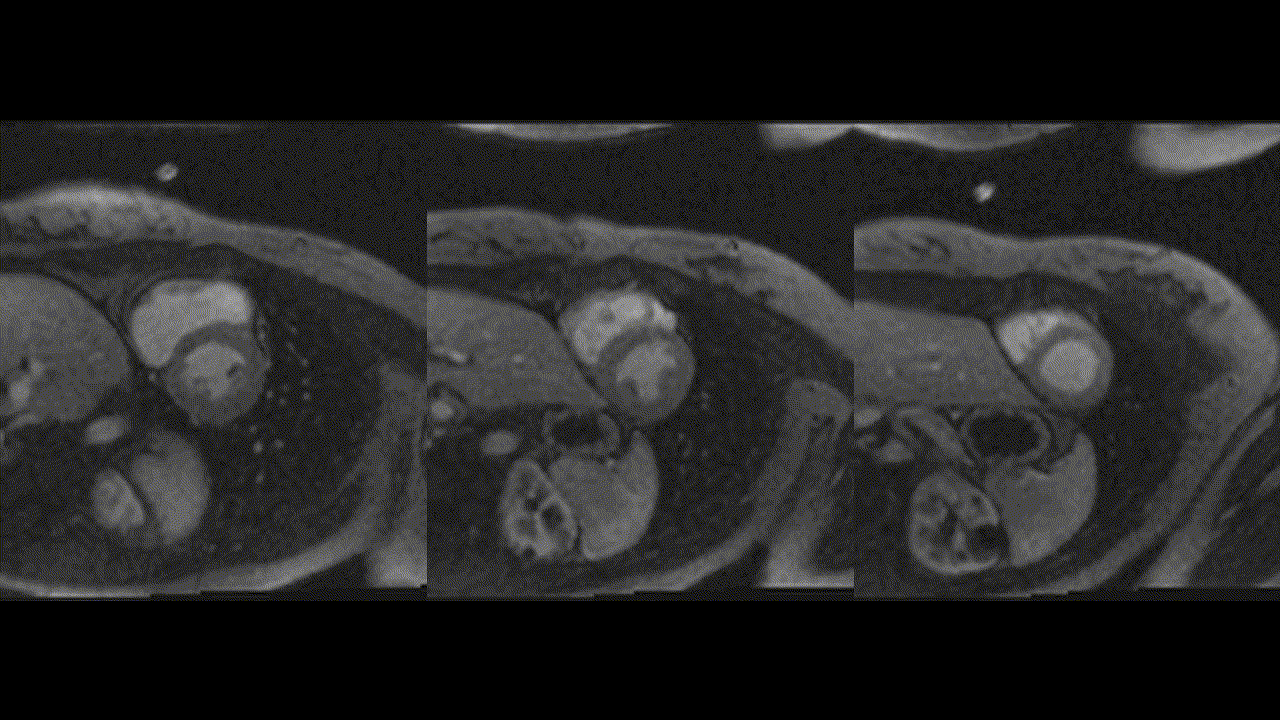
The LV end diastolic volume index was estimated to be 73 ml/m2, the LV mass index was estimated to be 64 g/m2 and the LV ejection fraction was estimated to be 57%. The RV size and systolic function was normal with the RV end diastolic volume index estimated to be 74ml/m2 and the RV ejection fraction an estimated 51%. However, tissue characterization sequences surprisingly revealed subtle but genuine sub-endocardial abnormalities. T2-weighted imaging showed somewhat diffuse sub endomyocardial oedema in mid to distal LV segments (Image 2). Rest perfusion sequences in short axis and apical 4-chamber view showed mid to apical sub-endocardial hypo-perfusion (Video 5, 6).
Image 2: T2-weighted (STIR) imaging of LV short axis extending from base to apex showing somewhat diffuse increase in subendocardial signal intensity suggestive of subendocardial edema.
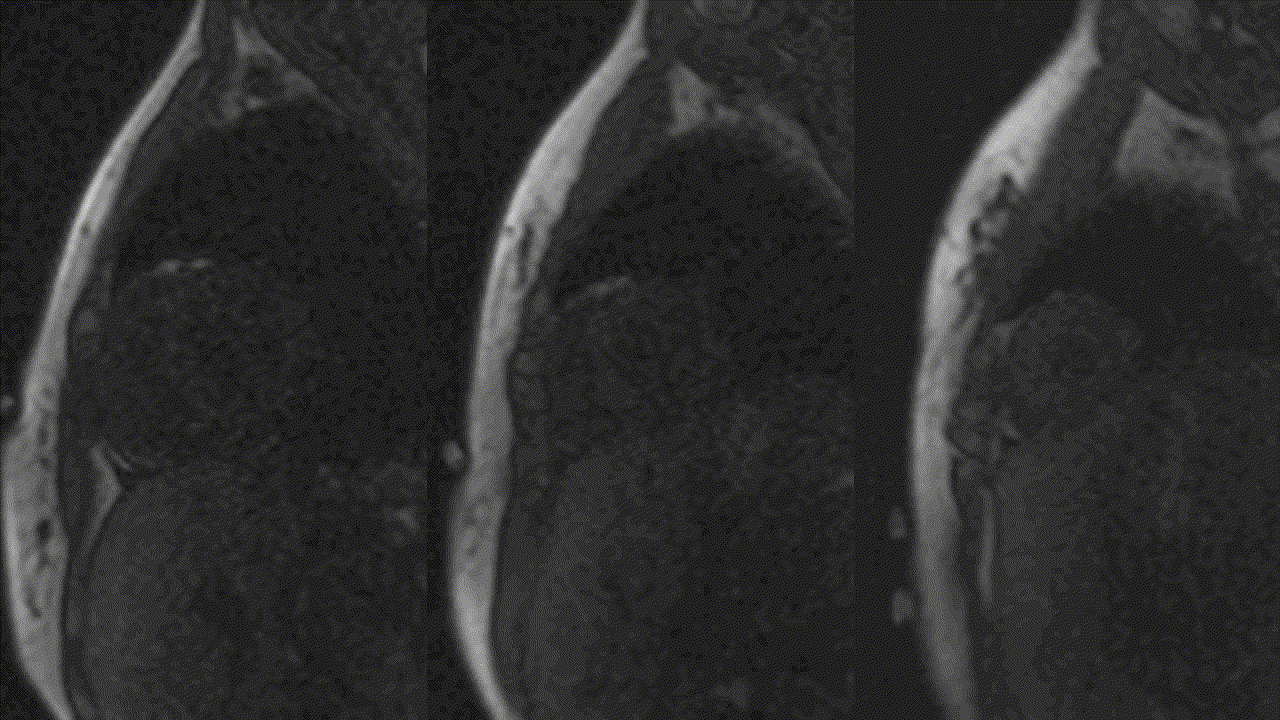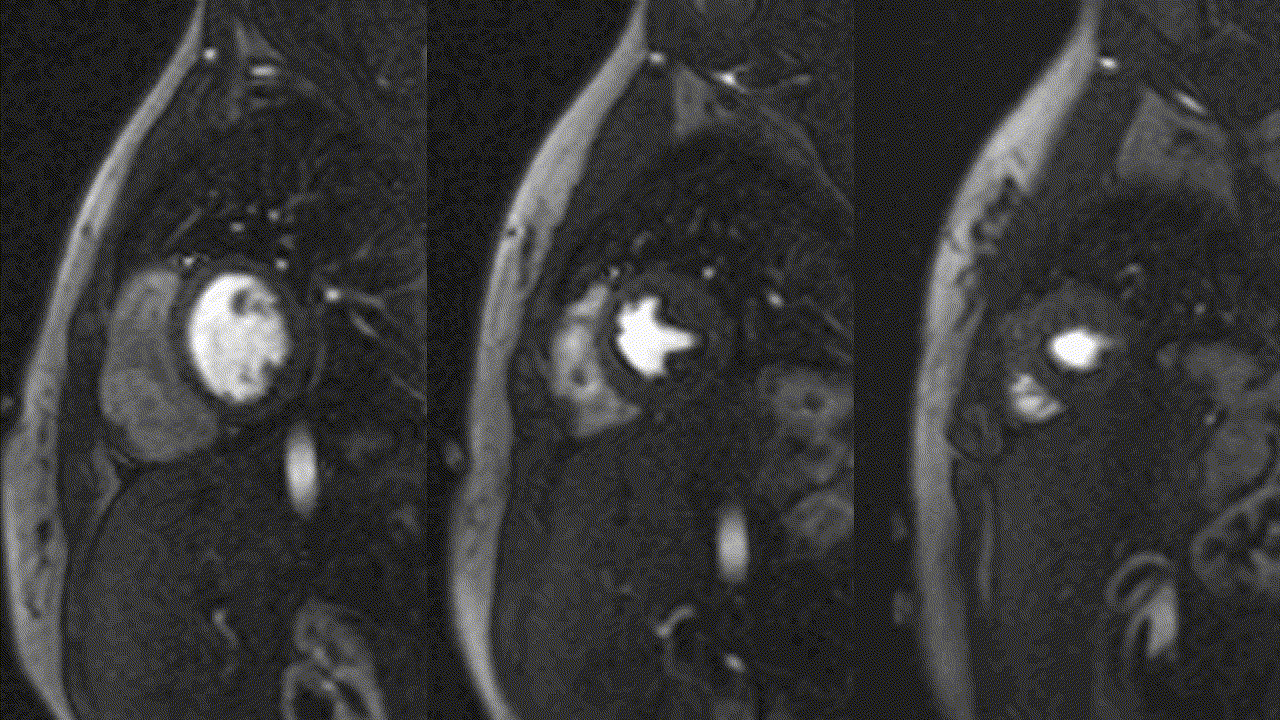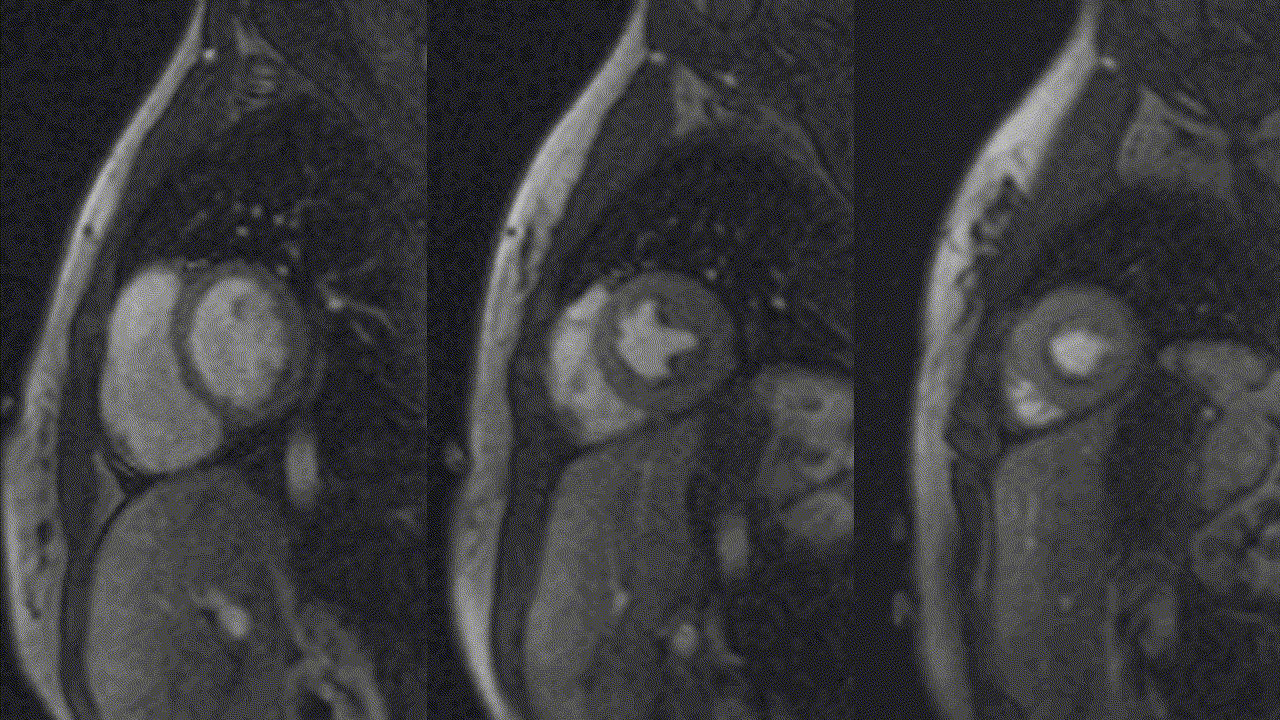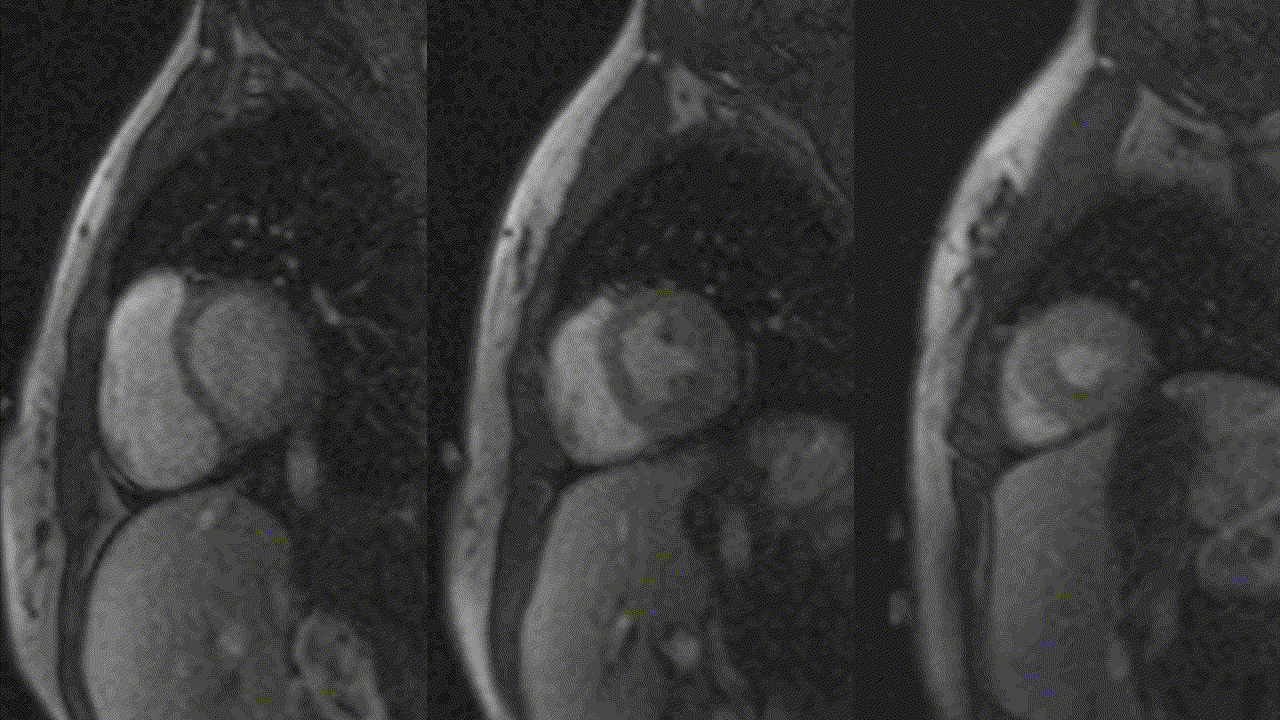
Video 5, 6: Rest perfusion imaging of LV basal-, mid- and apical- short axis, as well as 4-chamber views showing subtle subendocardial hypo perfusion predominately in mid- to apical- segments, likely related to microvascular dysfunction in context endomyocarditis secondary to eosinophilic infiltration.
Late gadolinium enhancement (LGE) imaging using T1-weighted imaging in short axis showed subendocardial enhancement in the mid to distal LV segments (Image 3). These findings correlated with LGE (PSIR) imaging in 3- and 4-chamber views (Image 4,5) also showing subtle subendocardial enhancement in the mid- and distal- LV segments.
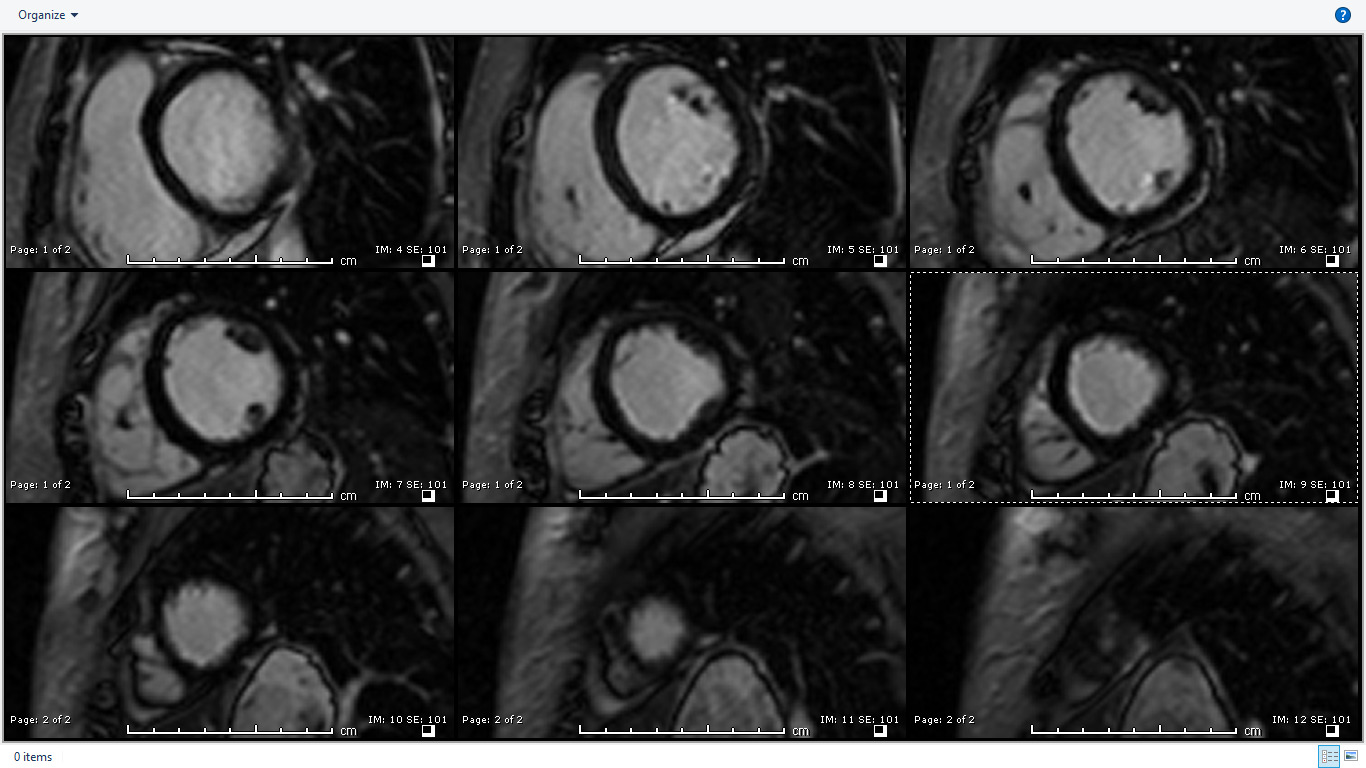
Image 3: T1 weighted (high resolution-magnitude) late gadolinium enhancement (LGE) imaging in short axis showing subendocardial enhancement in the mid to distal LV cavity.
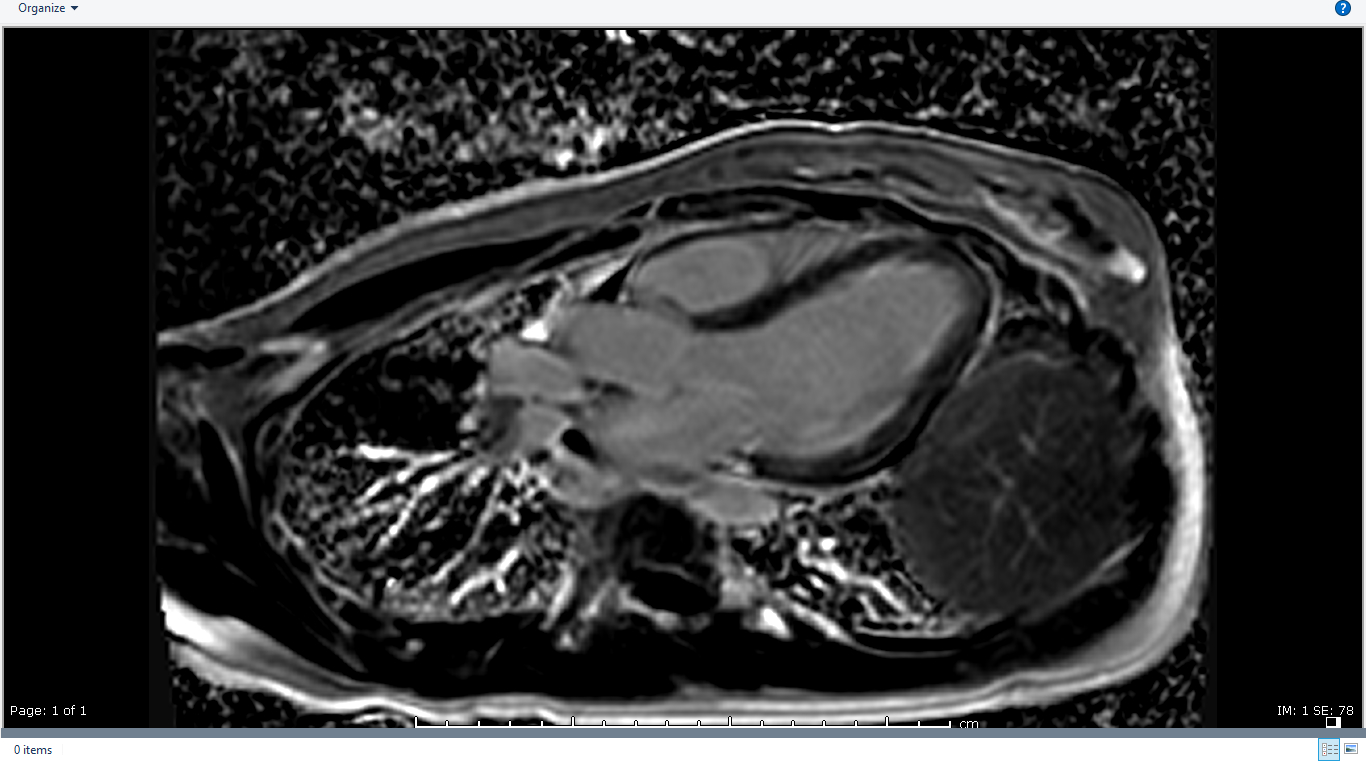
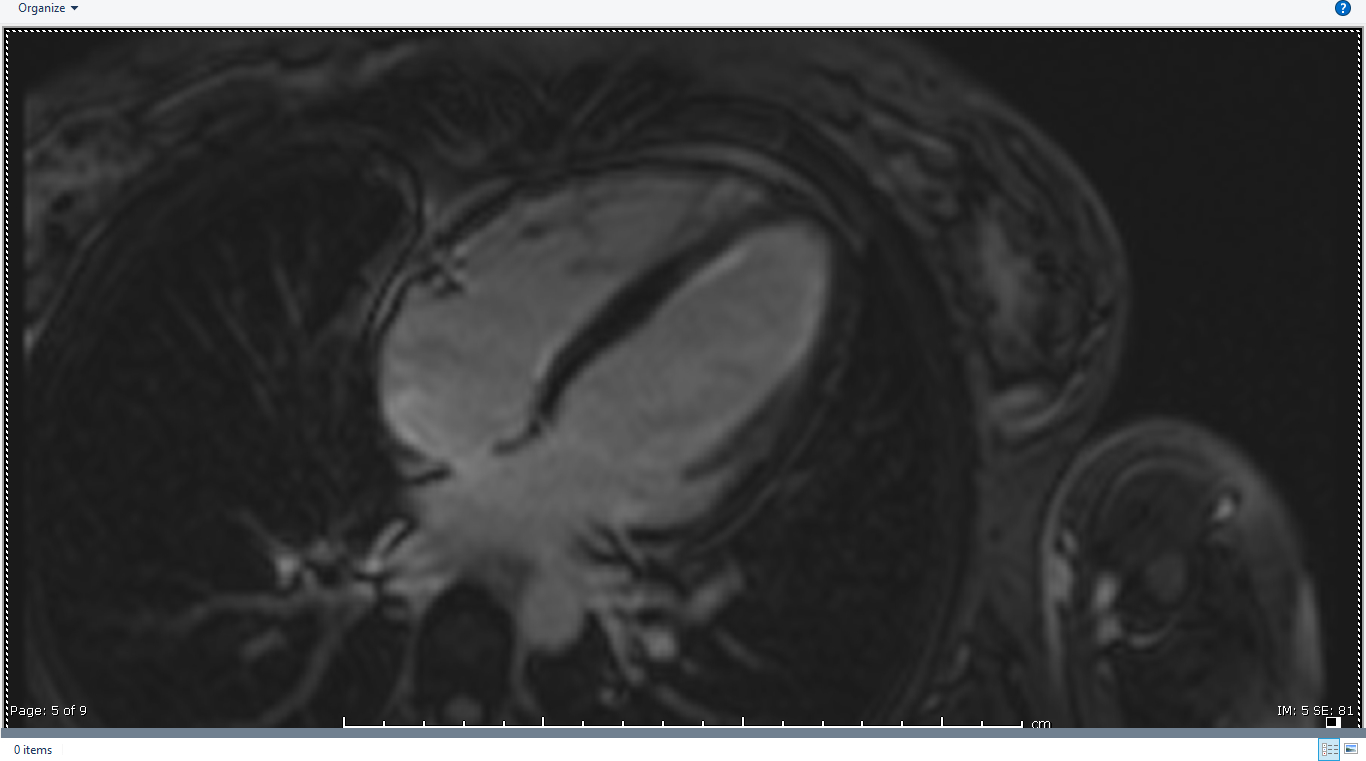
Image 4, 5: LGE (PSIR) imaging in 3- and 4-chamber views showing subtle subendocardial enhancement mid and distal LV segments.
Multi-parametric CMR: Pre- and post- contrast T1 mapping was performed on 3T Siemens using modified look locker inversion (MOLLI) sequence (Myomaps, Siemens healthcare). Region of interest involving apical septum had an elevated T1 of 1385 msec (normal range~ 1150-1300) as well as ECV of 39% (normal range ~ 20-30%). In the remote myocardium (uninvolved basal infero-septum) native T1 and ECV was in the normal range (1279) and ECV was within normal limits (25%) (Image 6a). Colored Maps also confirmed subendocardial elevation in pre-contrast T1 and ECV in mid to apical LV segments (Image 6b).
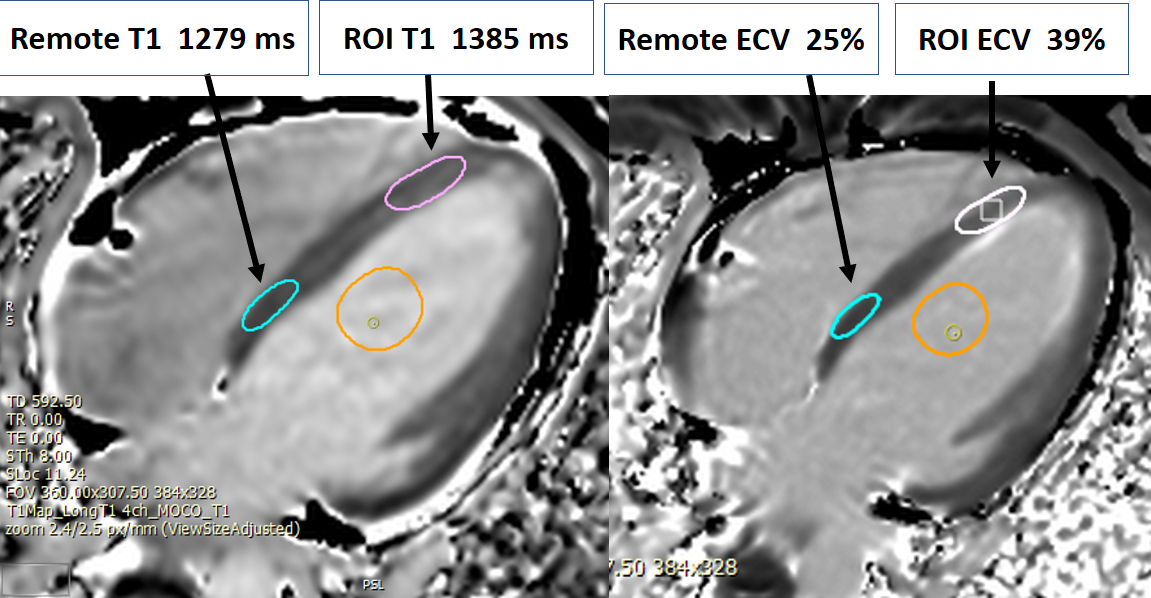
Image 6a: Pre and post contrast T1 and ECV color map in 4-chamber view.
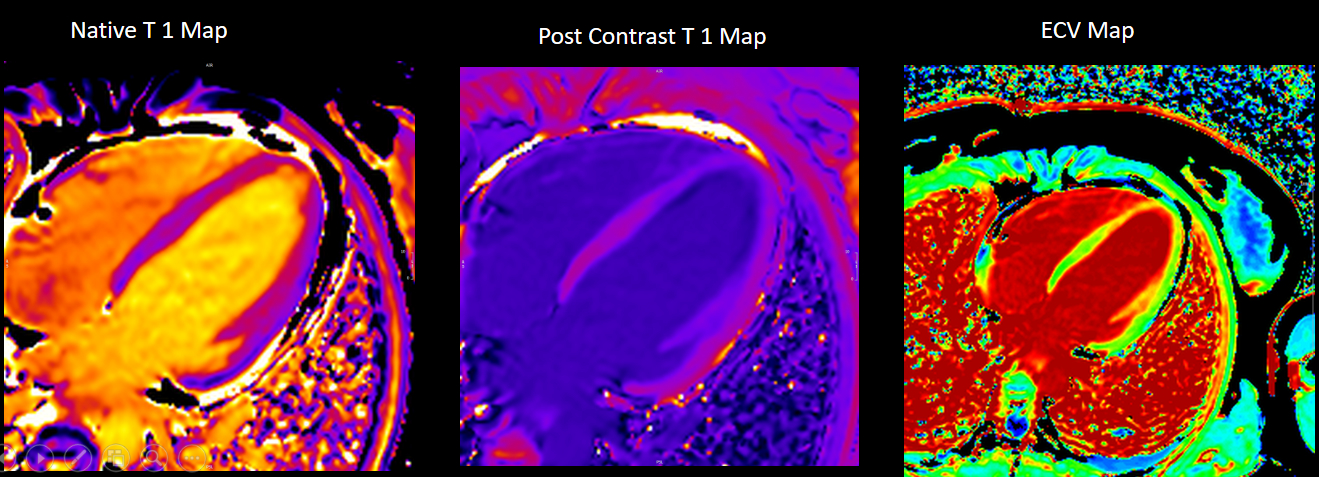
Image 6b: Pre T1 and ECV measured in region of interest (apical septum) and remote region (basal septum) in 4-chamber view.
The patient was subsequently investigated for a systemic disease such as eosinophilic granulomatosis with polyangitis (EGPA). Chest CT identified non-specific sub-pleural inflammatory changes (Image 7). CT guided needle biopsy of this lesion revealed eosinophilic infiltrates consistent with acute eosinophilic pneumonia with some vascular changes. Patient was subsequently treated with induction course of intravenous methyl prednisone followed by Rituximab with good symptomatic and biochemical response to therapy (follow up ESR 4, CRP of <2, resolution of eosinophilia).
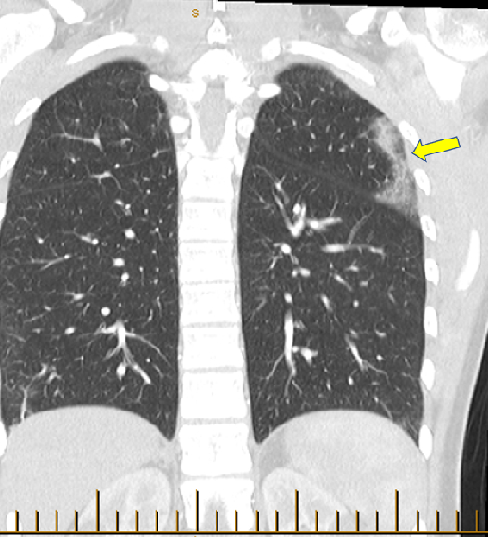
Image 7: Chest CT coronal view showing nonspecific sub pleural pulmonary infiltrates in the left upper lobe (arrow). CT guided needle biopsy of this lesion revealed eosinophilic infiltrates consistent with acute eosinophilic pneumonia.
Repeat CMR 6 months after treatment showed almost complete resolution of previously noted subendocardial abnormalities (Video 7,8 and Image 8a,b,c,d,e). Post treatment apical septum native T1 (1288ms) and ECV (30%) returned to normal range.
Video 7,8: Post-treatment resting perfusion imaging of LV base, mid and apical short axis, as well as 4-chamber view showing almost complete resolution of previously noted subtle subendocardial hypo perfusion.
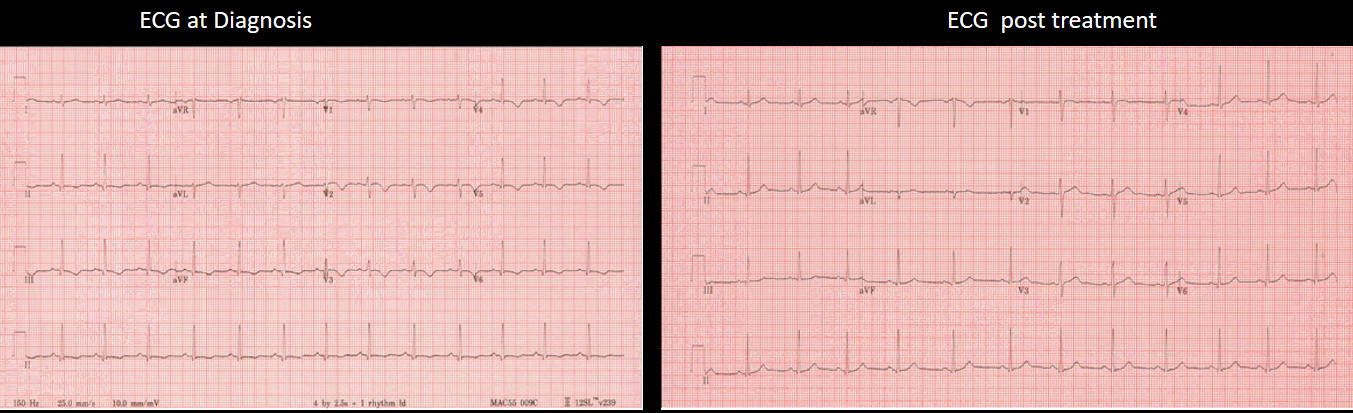
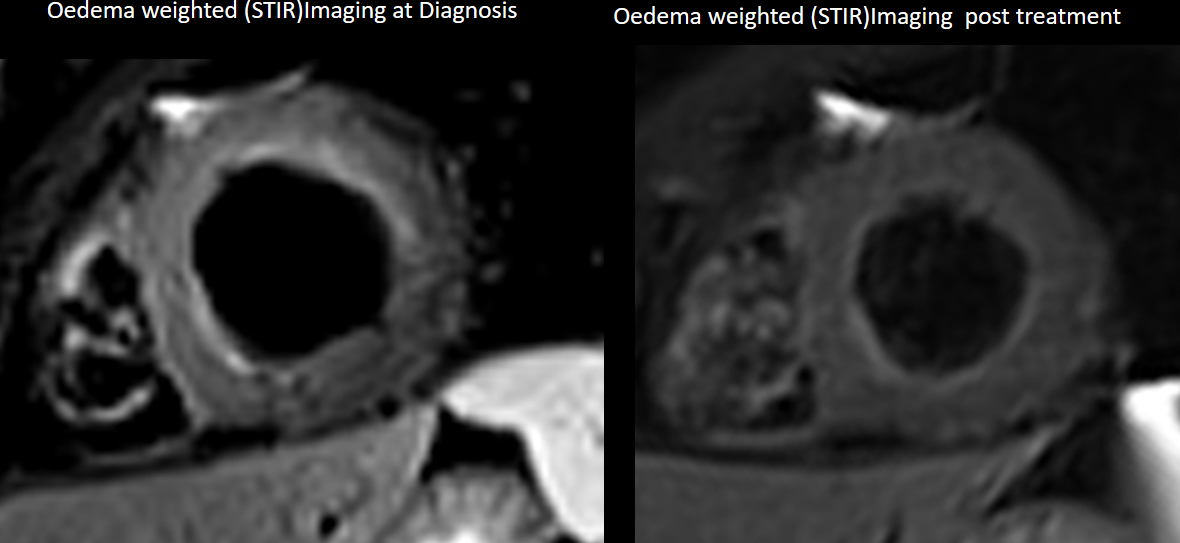
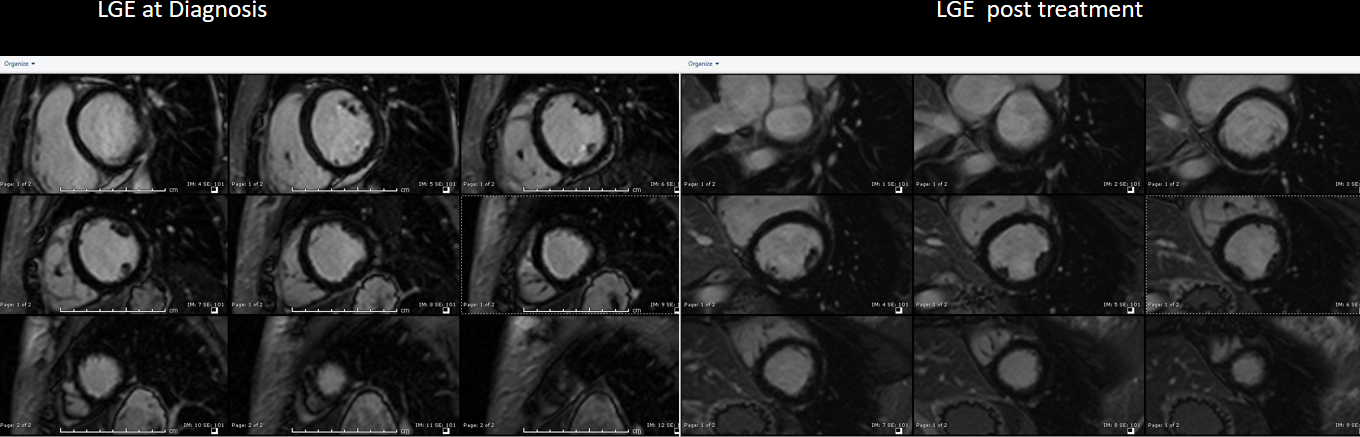
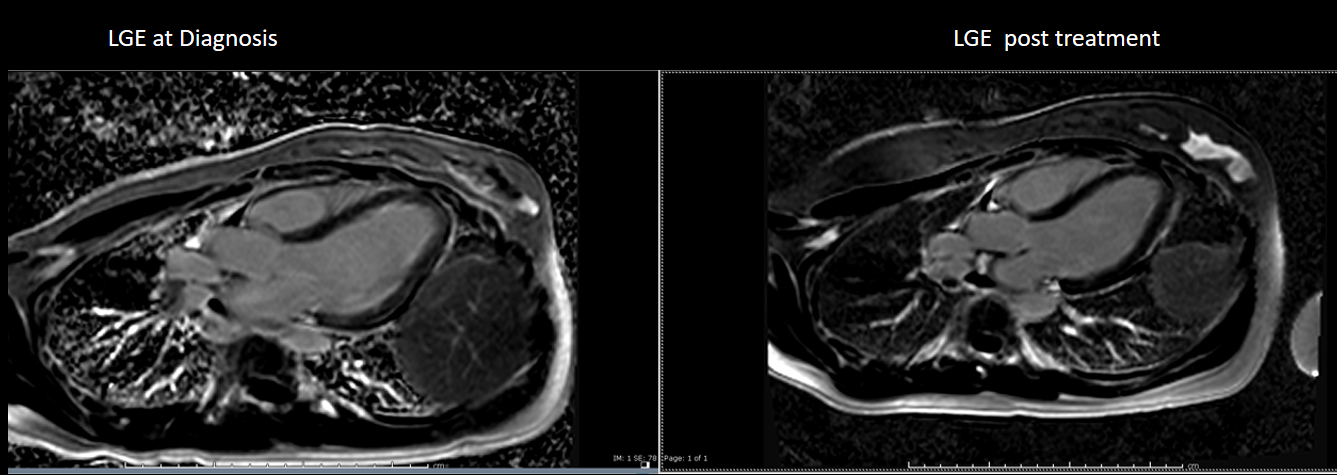
Image 8 (a,b,c,d,e,): Side by side comparison of pre- and post-treatment ECG, LGE images and edema weighted images showing almost complete resolution of previously described abnormalities.
Conclusion
In the context of the patient’s history, the findings on CMR were suspicious for endo-myocarditis related to early stage EGPA. CT chest revealed clinically silent pulmonary infiltrates with CT-guided biopsy of these lungs infiltrates confirming eosinophilic pneumonia, and therefore avoiding cardiac biopsy. The final diagnosis was EGPA causing lateral tarsal artery thrombosis, with respiratory involvement, sinusitis and asymptomatic cardiac involvement. Patient improved with immune suppression. Repeat CMR performed 6 months post treatment revealed significant interval resolution of abnormal MRI findings.
Perspective
Eosinophilic granulomatosis with polyangiitis (EGPA, previously known as Churg-Strauss syndrome), is a multisystem disorder characterized by chronic rhinosinusitis, asthma, and prominent peripheral blood eosinophilia (≥ 1500 cells/microL and/or >10 percent eosinophils on differential leukocyte count). The exact etiology of EGPA is unknown. Asthma is the cardinal feature (occurring in more than 95 percent of patients) and usually precedes the vasculitic phase by approximately 8 to 10 years.
American College of Rheumatology criteria for the classification of Churg-Strauss Syndrome requires at least four of the following six criteria: asthma, eosinophilia >10%, mononeuritis or polyneuritis, non-fixed pulmonary infiltrates, paranasal sinus abnormality and extravascular eosinophils (1) It is a rare condition with an estimated annual incidence of 1.0 to 4.2 people per million (2).
Cardiac involvement is one of the more serious manifestations of EGPA, accounting for approximately one-half of deaths attributable to EGPA. It should be suspected in the presence of refractory dyspnea, pericarditis related symptom, clinical evidence of heart failure, or cardiac rhythm abnormalities, but can also be asymptomatic. Endomyocarditis may represent the most severe manifestation with eventually fatal outcome. A structured clinical assessment incorporating cardiac imaging with echocardiography and MRI can identify impaired cardiac function and endomyocardial abnormalities (3). CMR is superior to echocardiography revealing cardiac abnormalities in 62% of Churg-Strauss patients compared to 50% by echocardiograms in one study (4). Furthermore in this study, the absence of symptoms or ECG abnormalities did not exclude cardiac involvement, because abnormalities could still be detected in 38% of these patients at the time of CMR.
CMR is a useful tool for the differential diagnosis of HCM, as it can differentiate HCM from other mimicking conditions because of its superior spatial resolution and its ability to tissue characterise. Tissue characterisation by CMR can differentiate eosinophilic endomyocarditis from apical HCM. Unlike apical HCM which usually has midmyocardial late gadolinium enhancement of apex, eosinophilic endomyocarditis typically has diffuse subendocardial enhancement, often associated with thrombus (5). Furthermore this case highlights that CMRis more sensitive than echocardiography in detecting cardiac involvement in Churg strauss and occasionally CMR can provide important clues and contribute towards the EPGA diagnostic criteria. Finally, early diagnosis by CMR in this case lead to early immunosuppressive treatment and prevented potentially catastrophic complications.
Please click here to view the case on CloudCMR.
References
Case Prepared by:
Madhusudan Ganigara, MD
Associate Editor SCMR Case of the Week.
Cohen Children’s Medical Center of New York, Hofstra-Northwell School of Medicine.







