Sweeney, A; Hamilton-Craig, C; Katikireddi, S; Wesley, AJ
Department of Cardiology, The Prince Charles Hospital, Brisbane, QLD, Aust
Department of Rheumatology, The Prince Charles Hospital, Brisbane, QLD, Aust
Department of Medical Imaging, The Prince Charles Hospital, Brisbane, QLD, Aust
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical history
A 23-year-old migrant from Zimbabwe presented with a 10 day history of flu-like symptoms, myalgias, chest pain, palpitations, Troponin I of 1.8 mcg/L (normal <0.040) and peripheral eosinophilia of 3.42 x109/L (normal <0.6 x109/L). He had been diagnosed with anti-neutrophil cytoplasmic antibody (ANCA) negative eosinophilic granulomatosis with polyangiitis (EGPA) with cardiac involvement 2 years prior, in 2015. Having been managed well on oral methotrexate 25mg weekly and weaning dose of oral prednisolone, relapse occurred once his prednisolone was decreased to 4mg daily.
Cardiac investigations were carried out including ECG which demonstrated normal sinus rhythm, normal axis, no PR depression. Echocardiogram showed a mildly dilated left ventricle with normal systolic function, ejection fraction (EF) by 3D estimation was 67%. Trivial loculated pericardial effusions without evidence of haemodynamic compromise (global longitudinal strain (GLS) = 21%, right ventricular systolic pressure (RVSP) = 21mmHg). Endomyocardial biopsy had been carried out in 2015 and was normal, showing no morphological abnormality, no fibrosis or fibrin. No myocyte damage or necrosis was noted. No granulomata or giant cells and no evidence of vasculitis.
Ultimately, his diagnosis of EGPA was made in 2016 following extensive investigation when he presented with dyspnoea, chest and abdominal pain on a background of adult onset asthma. He was found to have bilateral pleural effusions and a raised peripheral blood eosinophil count. Diagnosis was ultimately made based on biopsy of skin lesions (which developed in early 2016) and gastrointestinal tract which demonstrated an eosinophilic vasculitis pattern. Cardiac involvement at initial presentation included pericardial effusion as outlined above.
CMR findings
CMR imaging (1.5 Tesla, multiplanar cine steady state free precession, T2 double inversion recovery imaging with and without fat saturation and multi-planar late gadolinium enhancement imaging) was carried out at first presentation in 2015 showing a moderate pericardial effusion (image 1), without definite myocardial oedema or enhancement. EF was maintained at 53%. This was followed by a normal cardiac biopsy with no evidence of vasculitis/necrosis or fibrosis.
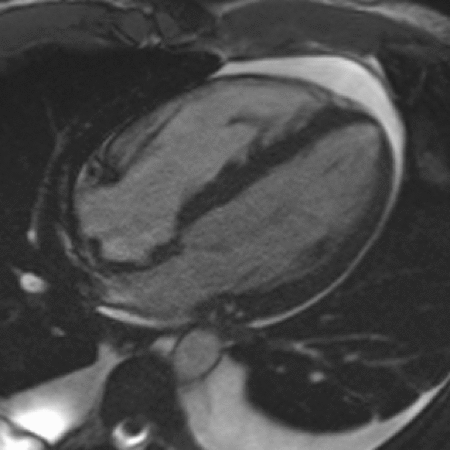
Image 1. Cine SSFP sequence from initial MRI with moderate pericardial effusion.
CMR was repeated in April 2018 upon re-presentation which now showed multifocal myocardial hyper-intensity in the basal to mid anterior, lateral and inferior left ventricular (LV) walls on T2 double inversion recovery (DIR) sequences suggesting oedema (image 2 – A three image composite of sequential basal and a mid LV short axis images with a narrow window) and multifocal midwall and sub-epicardial delayed gadolinium enhancement scattered throughout the LV myocardium, particularly the lateral LV wall (image 3). Relative sparing of the apical segments was noted. There was a small pericardial effusion without significant pericardial thickening or pericardial enhancement with no evidence of ventricular interdependence. Interval resolution of the bilateral small pleural effusions had occurred. (image 4) Overall, the findings were consistent with myocarditis.
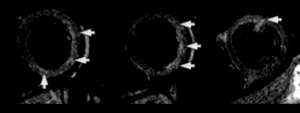
Image 2. T2 double inversion recovery (DIR) sequences suggesting oedema.
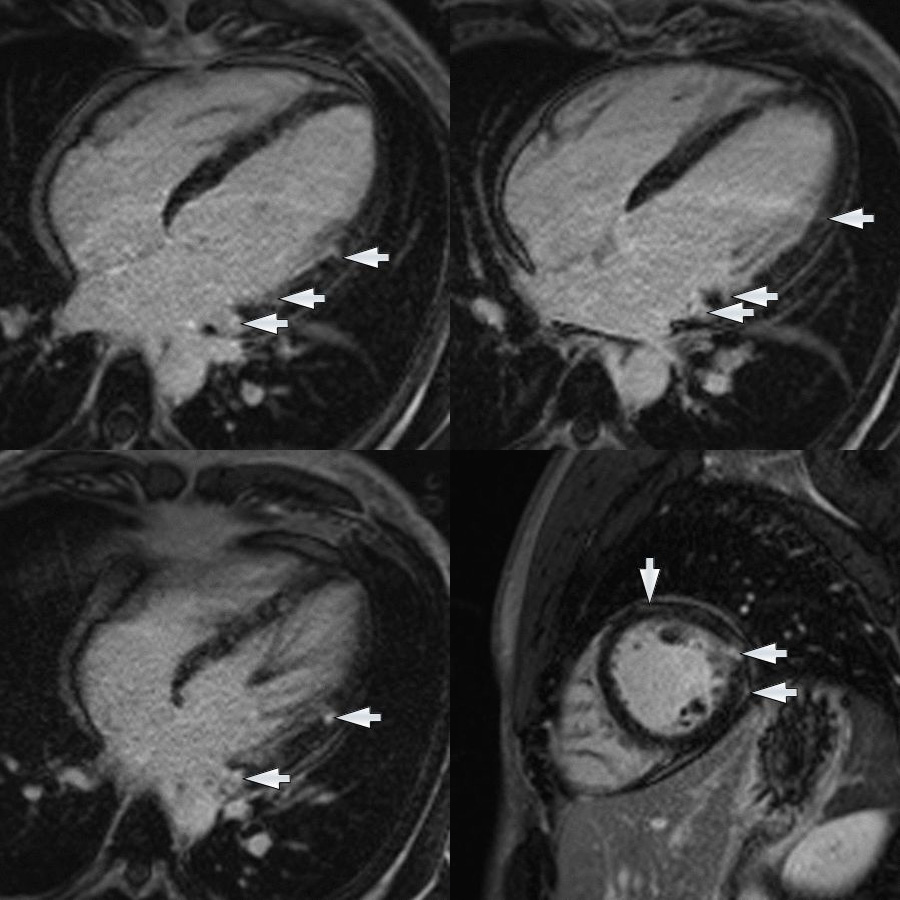
Image 3. Multifocal late gadolinium enhancement throughout the left ventricular myocardium, particularly the lateral left ventricle.
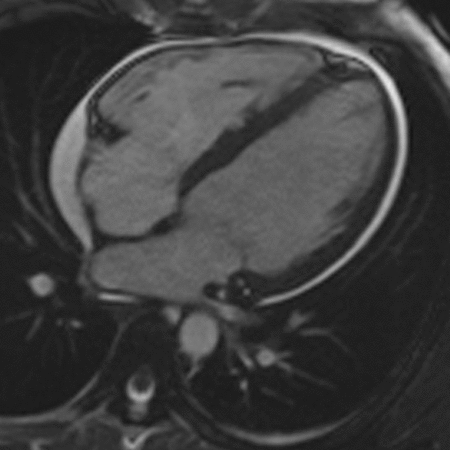
Image 4. SSFP cine image demonstrating mall pericardial effusion without significant pericardial thickening or pericardial enhancement. There was no evidence of ventricular interdependence. Interval resolution of the bilateral small pleural effusions had occurred
Conclusion
This case highlights the importance of CMR in the diagnosis of myocardial involvement in a biopsy negative case of EGPA.
Myocardial involvement was proven on CMR when echo findings were unhelpful. It also avoided the requirement for repeat cardiac biopsy thereby obviating the necessity for an invasive procedure. Accurate diagnosis allowed for appropriate prognostication and ultimately guided therapy. The patient was subsequently treated with intravenous methylprednisolone and rituximab therapy with excellent clinical response over 6 months and continues to be followed up under the care of rheumatology.
Perspective
EGPA (previously known as Churg-Strauss vasculitis) is a rare, vasculitic condition which can affect small and medium sized arteries. It is associated with adult onset asthma, allergic rhinitis and a peripheral blood eosinophilia. It can be associated with multi-organ involvement including cardiac as demonstrated above. Approximately 40-60% of patients with EGPA have positive ANCA (1). ANCA negative patients, as in this case, tend to exhibit more eosinophil tissue infiltration with higher incidence of myocardial involvement and pulmonary infiltrates. Coronary arteritis and myocarditis are the main causes of morbidity and mortality (2).
Findings of myocarditis on CMR can include increases in T2 signal intensity and increased native myocardial T1 mapping relaxation times consistent with myocardial oedema, increase in early myocardial contrast enhancement relative to skeletal muscle consistent with hyperaemia, and presence of late gadolinium enhancement consistent with necrosis or scarring (3). CMR allows for tissue characterisation in myocarditis specifically diagnosing local oedema and demonstrating late gadolinium enhancement. Specificity of myocarditis diagnosis by CMR is as high as 91% in some studies and this non-invasive technique may be used in clinical context to avoid invasive cardiac biopsy (4). Lake Louise Criteria may assist in the diagnosis of myocarditis. At least one T2 based criterion, global or regional increase of myocardial T2 relaxation time or an increased signal intensity in T2 weighted CMR images with one T1 based criterion, increased myocardial T1 time, extracellular volume, or late GAD enhancement is required for diagnosis. Of note however, discrete lesions may be more difficult to detect after the first few days as the T2 hyperintensity signal becomes gradually more homogenous as oedema and inflammation becomes more diffuse within the myocardium (4).
Cardiac involvement in EGPA is associated with poor prognosis. Prognostication is based on the number of organs involved and scoring systems such as the five factor score rely heavily on this for prognostication and to guide treatment (5).
CMR was pivotal in this particular case as diagnosis of myocardial involvement demonstrated a changing clinical picture and allowed for prognostication in the setting of relapsing EGPA. This ultimately highlighted failure of maintenance therapy and guided treatment response.
References
Case prepared by
Eddie Hulten, MD MPH FACC FSCMR
Associate Editor, SCMR Case of the Week







