Joseph Burdowski, MD1; Ravi Marfatia, MD1; Xiaoming Bi, Ph.D2; Jason Craft, MD1
1. St Francis Hospital, Roslyn NY, USA
2. Siemens Medical Solutions USA, Inc., Los Angeles CA, USA
Case Published in the Journal of Cardiovascular Magnetic Resonance: Click here for the link
Click here for PubMed Reference to Cite this Case
Clinical History:
A 57 year old female with a past medical history of eosinophilic granulomatosis with polyangiitis (EGPA), Loeffler’s endocarditis (LE), asthma, hypertension, and syncope with an implantable loop recorder presented for a follow up cardiac MRI to assess for disease progression and response to therapy. She has had known EGPA since 2007 and has had multiple prior cardiac MRI studies, which have been significant for biventricular subendocardial enhancement with overlying thrombus. Our patient has been chronically anticoagulated with warfarin and on chronic oral prednisone therapy since her initial diagnosis. Due to progression of myocardial eosinophilic infiltration noted on cardiac MRI in 2018, she was started on mepolizumab (Nucala) 300mg once every 4 weeks.
Upon presentation for the current study, her ECG demonstrated normal sinus rhythm and a right bundle branch block. No recent laboratory testing was available for review as the patient follows with hematology at a separate institution.
CMR Findings:
CMR imaging was performed approximately one year prior in January 2019 on a 1.5 Tesla scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany), showing subendocardial fibrosis within the left and right ventricular apices, extending to the mid-ventricular level. There was also enhancement of the basal anterolateral subendocardium. (Figure 1).
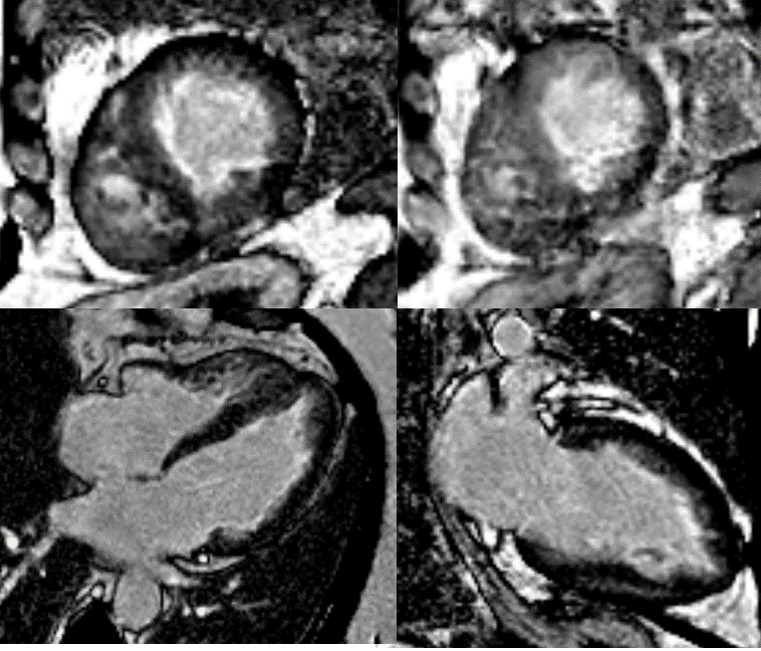
Figure 1. Late gadolinium enhancement images acquired with GRE readout and PSIR reconstruction from prior MRI (2019) with subendocardial enhancement in the left and right ventricular apices, extending to the mid-ventricular level, plus of the basal anterolateral subendocardium.
CMR was repeated in February 2020 at 1.5 Tesla (MAGNETOM Sola; Siemens Healthcare, Erlangen, Germany) for reassessment. The left ventricular ejection fraction was 50% and the right ventricular ejection fraction was 52%. The burden of late gadolinium enhancement extended to the mid-ventricular level, with also involvement of the basal anterolateral subendocardium (Figure 2). Left ventricular thrombus was not identified, and this was also not present on the prior exam. Using blood pool nulling, the areas of subendocardial enhancement were more clearly demonstrated due to the increased contrast to noise ratio between fibrotic regions and the left ventricular cavity blood pool compared to the prior exam from 2019. The magnitude images from the current study demonstrate the use of blood pool nulling (Figure 3).
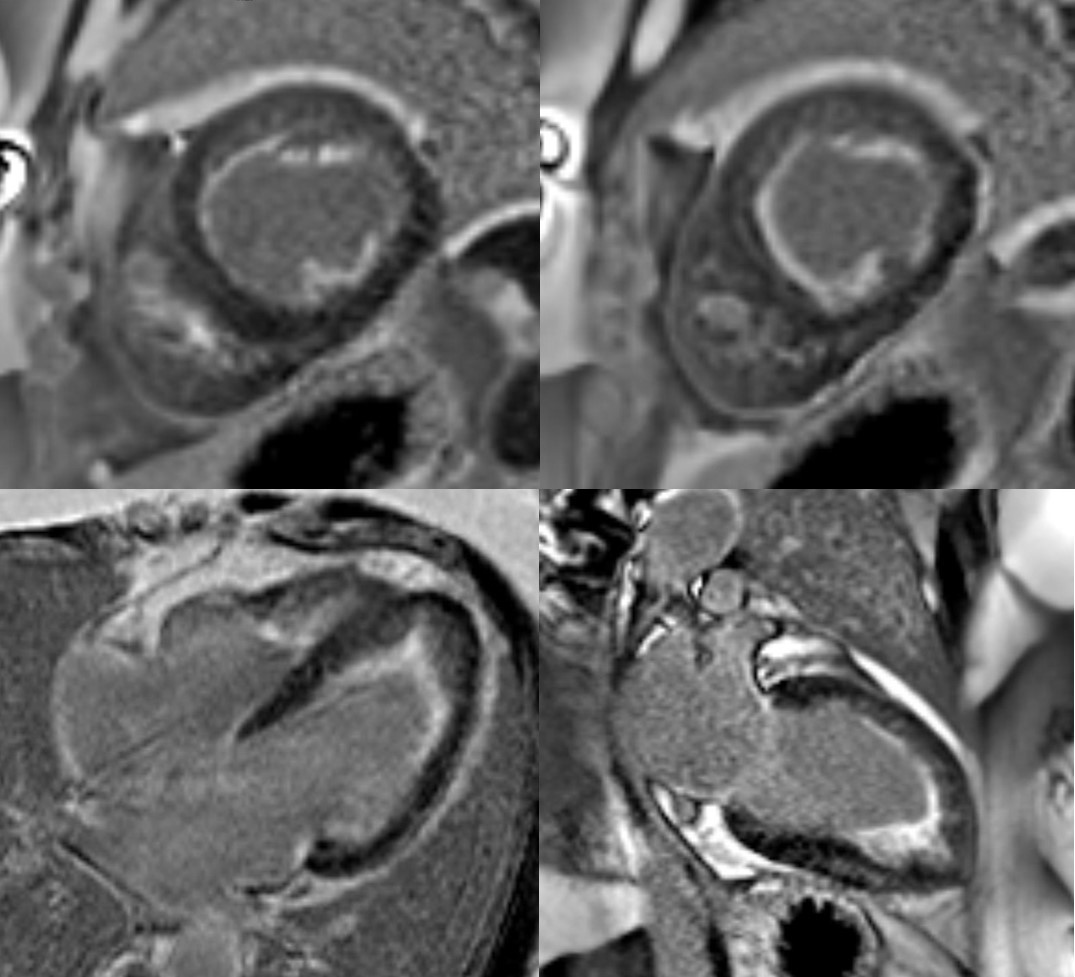
Figure 2. PSIR late gadolinium enhancement on the current exam. There is subendocardial enhancement in the left and right ventricular apices. Note the improved contrast to noise (CNR) between the fibrotic layer and the left ventricular cavity blood pool when blood pool nulling is used.
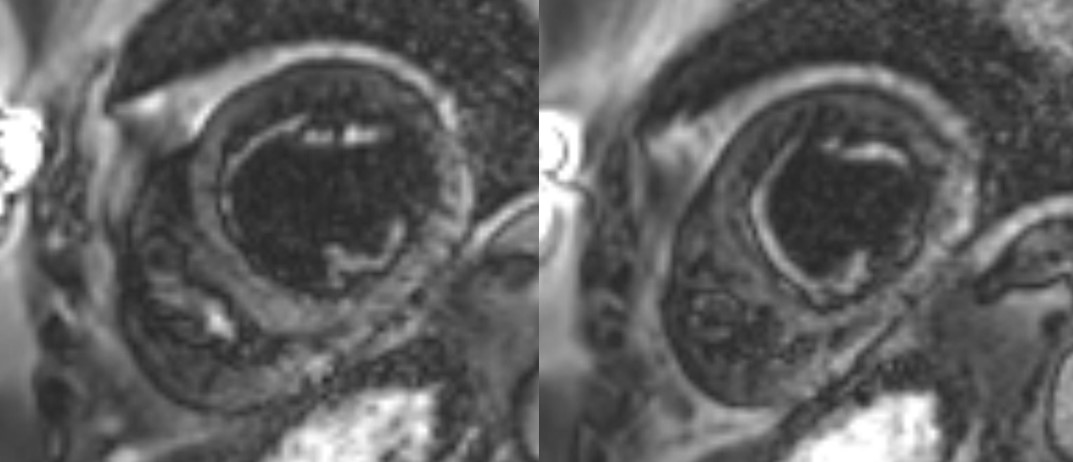
Figure 3. Magnitude short axis images corresponding to PSIR reconstruction from figure 2. This demonstrates the blood pool nulling technique.
A multi-echo GRE fat-water separation sequence demonstrated the presence of subendocardial fat in both ventricles which can be seen in the out of phase and fat only images (Figure 4a-4d). This finding can also be appreciated on bSSFP cine imaging (Figure 5a, 5b). On first pass perfusion, there were defects at the subendocardial layer (Figure 6a, 6b) corresponding to extensive fibrosis within the left and right ventricular apices.
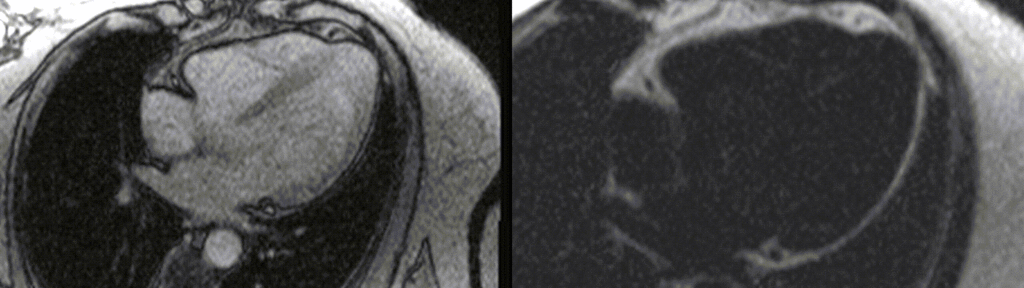
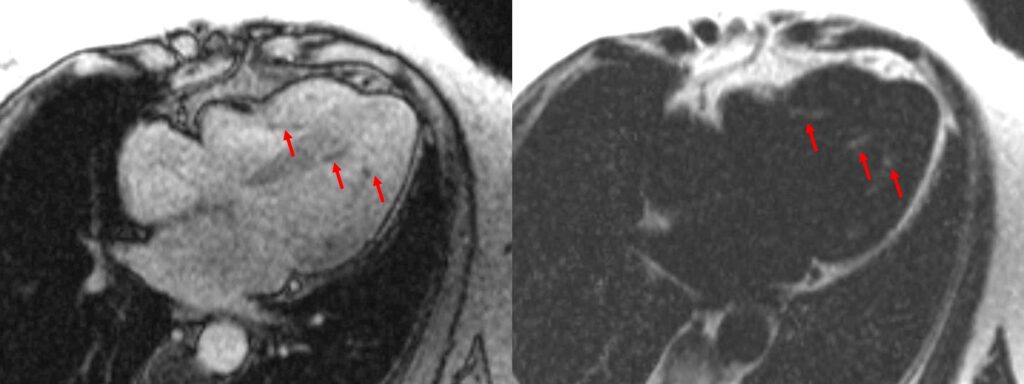
Figure 4. Images from multi-echo GRE fat water separation sequence: out of phase cine (a) and fat only cine (b). Subendocardial fat deposition is depicted by the arrowheads (c and d).
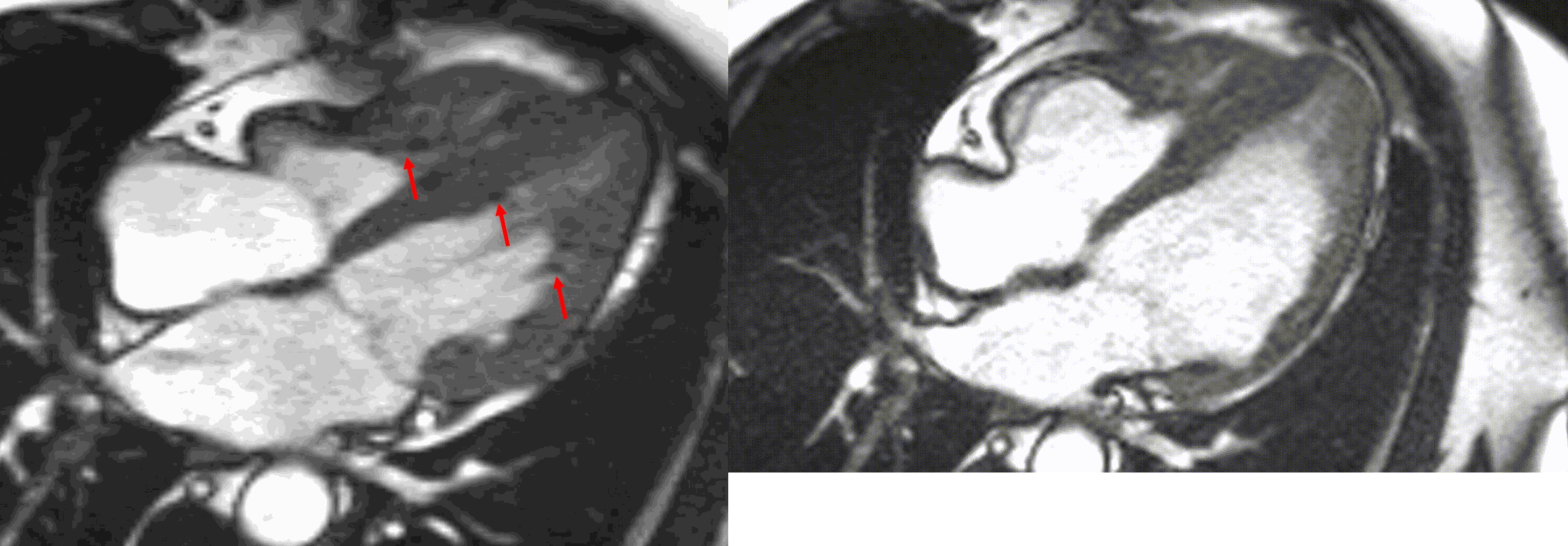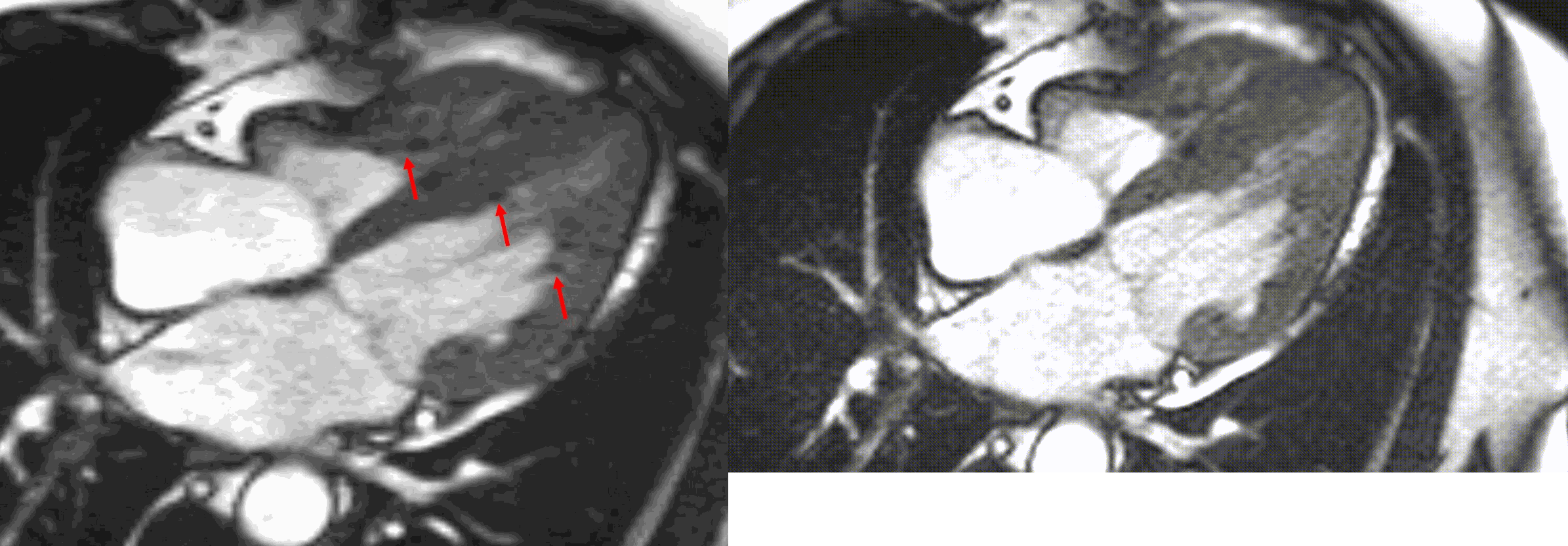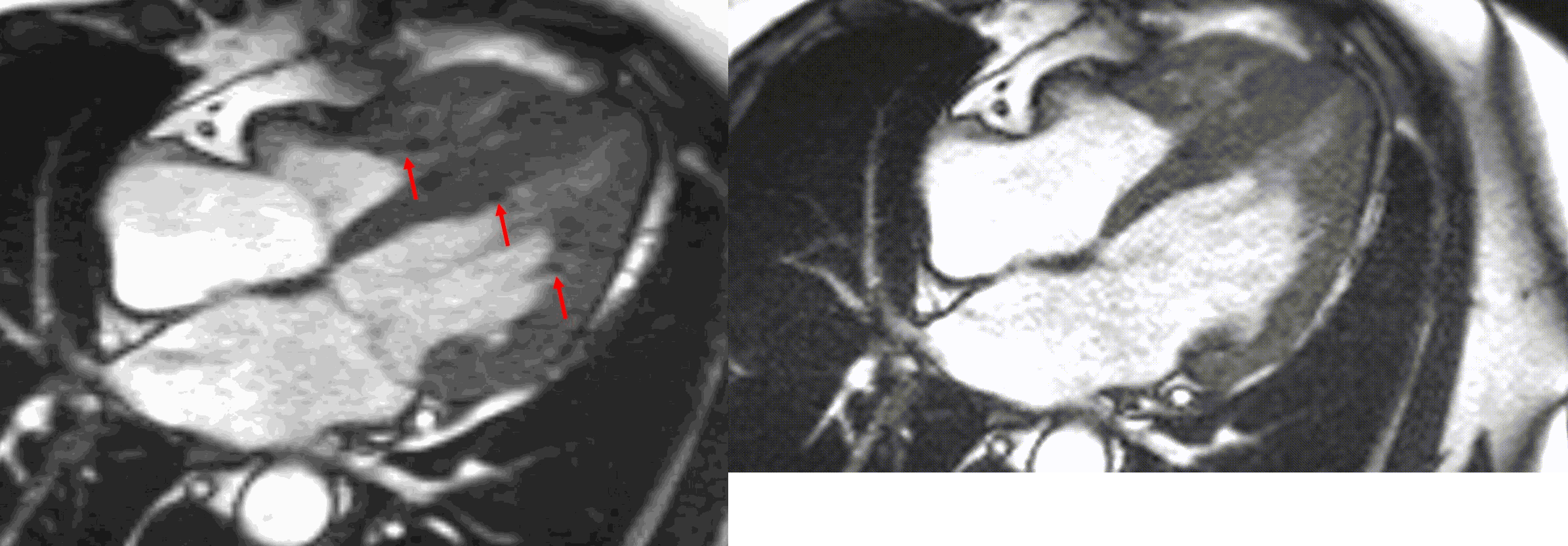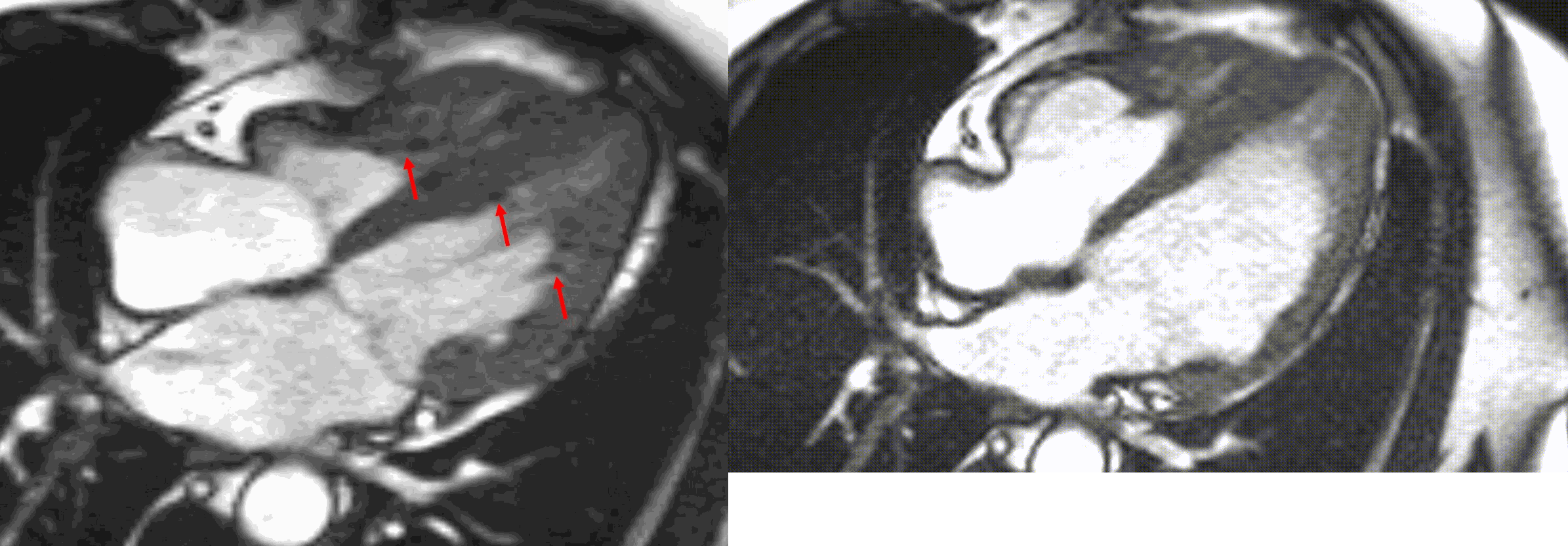
Figure 5. TrueFISP cine images from current MRI demonstrating hypointense regions within the subendocardium corresponding to scattered areas of fat deposition: (a) still image with arrows, (b) corresponding cine image.
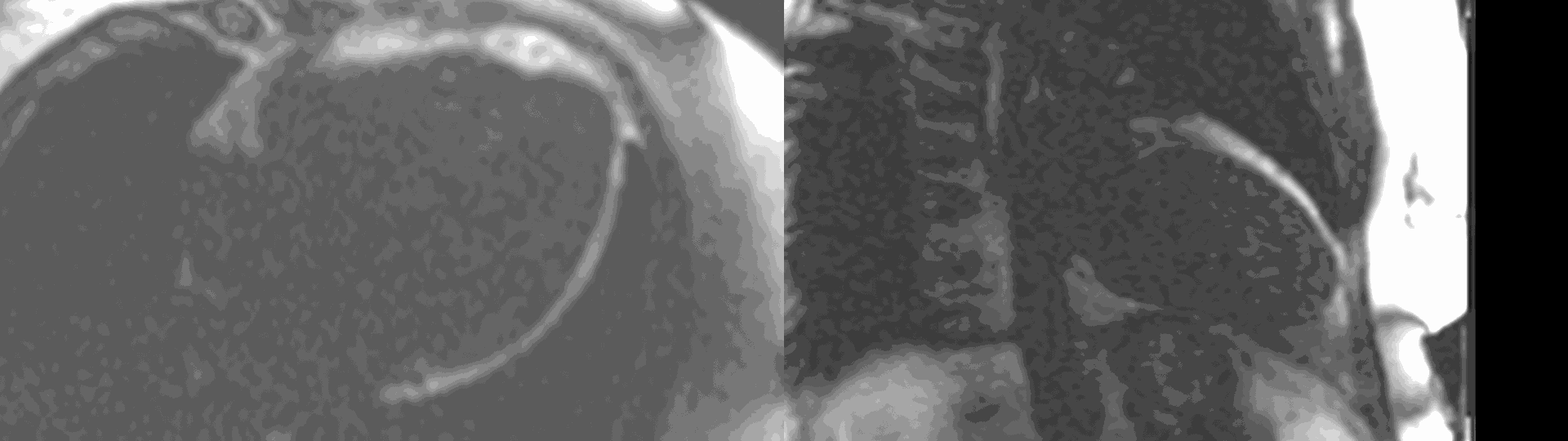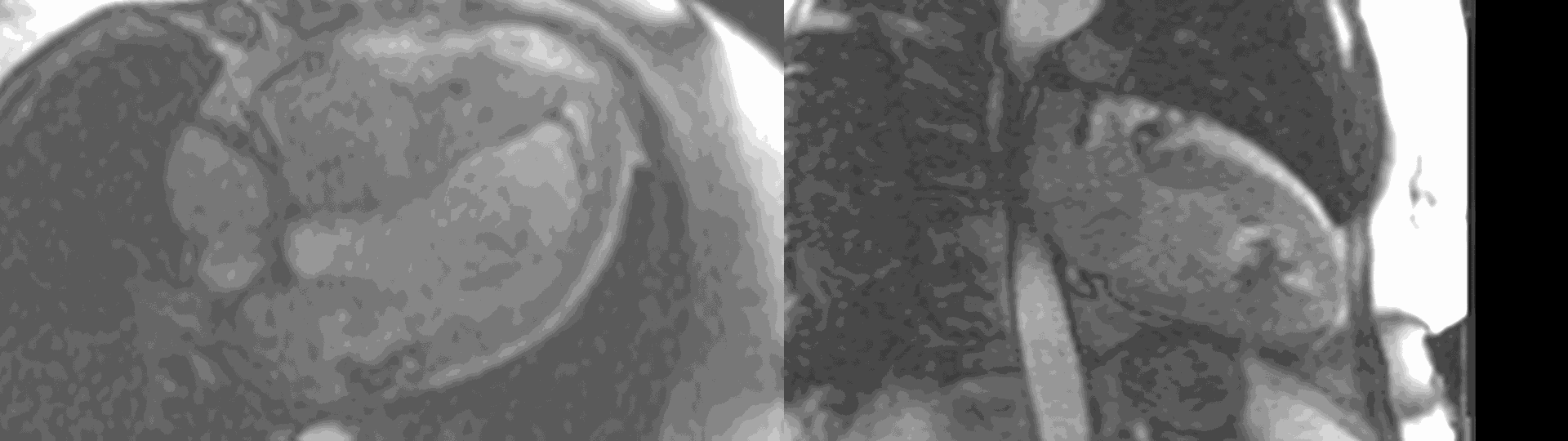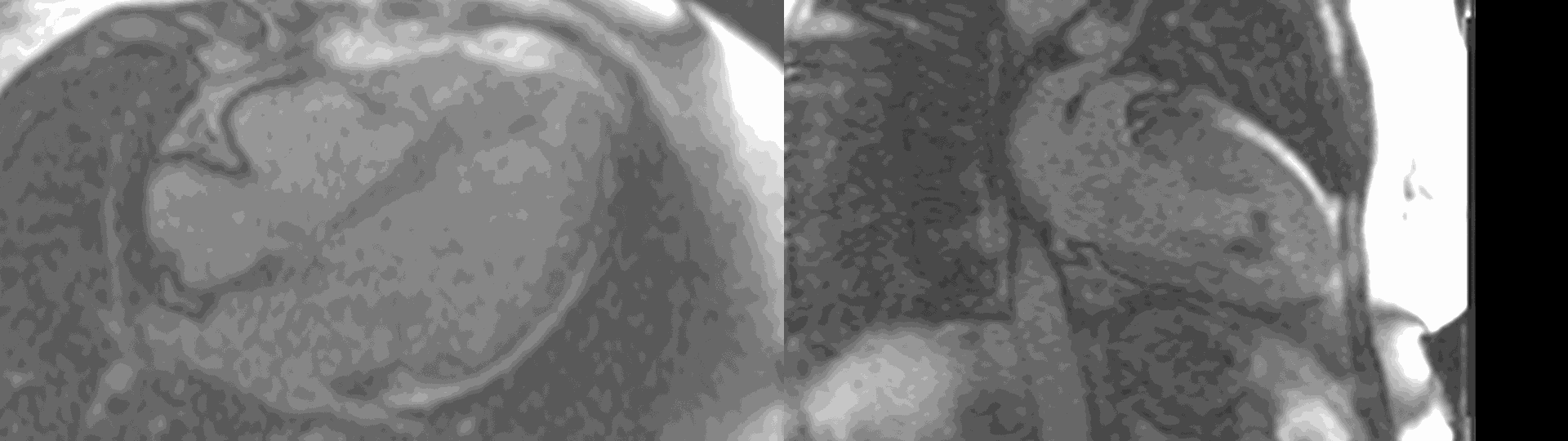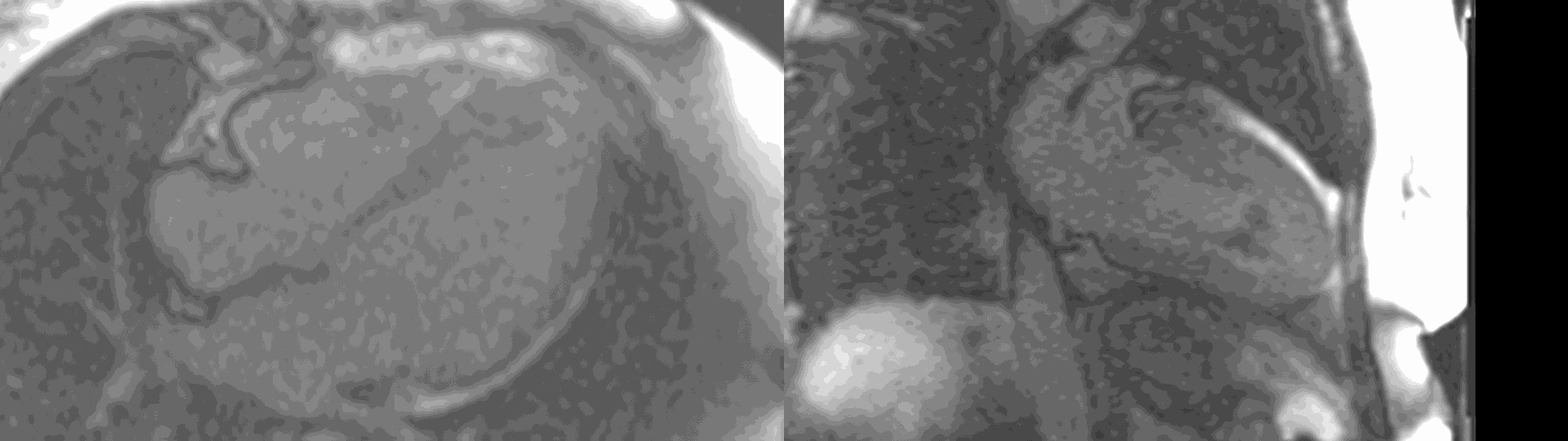
Figure 6. First pass perfusion imaging in the 4 chamber (a) and 2 chamber (b) views showing the subendocardial perfusion abnormality which corresponds to areas of LGE.
Conclusion:
The presence of subendocardial fat, as seen in this case, has not been previously described in LE associated with EGPA. We postulate that this may represent an atypical feature of the disease or alternatively represent a finding unrelated to the disease process of LE. This finding is unlikely to represent artifact as the phenomena was demonstrated on images acquired with multiple sequence types. The case underscores the important role of CMR for tissue characterization in various disease processes. The use of multi-echo based fat-water separation techniques overcomes the limitations of spectral frequency based fat saturation. Additionally, using double/triple inversion spin echo, abnormalities at the myocardial-blood pool interface may be obscured due to incompletely suppressed blood. The Dixon technique exploits phase cycling between fat and water which completes approximately every 4.4 milliseconds at 1.5 Tesla. At this field strength, the resonant frequencies of fat and water are separated by 220 hz. Therefore, at an echo time of approximately 2.2 seconds, fat and water are at opposite phase (separated by ½ cycle or 180 degrees), but are in-phase relative to one another at 4.4 milliseconds. Fat only, and water only images can be reconstructed based on this principle (1). Finally, the use of blood pool nulling can improve the conspicuity of fibrosis at the left ventricular blood pool/subendocardial interface to markedly improve visualization of subendocardial enhancement.
Perspective:
EGPA is a systemic small to medium vessel vasculitis, associated with asthma and peripheral eosinophilia (2). Though primarily involving the upper respiratory tract, multi-organ involvement is often described. Cardiac manifestations can be seen in up to 62% of patients and include myocarditis, heart failure, acute coronary syndrome, pericarditis, or pericardial effusion (3). The three stages of myocardial involvement due to hypereosinophilia include an initial necrotic phase, followed by thrombus formation and ultimately endomyocardial fibrosis.
The finding of fat deposition seen on fat water separation imaging has not been described in the literature with Loefflers’ endocarditis, although it has been documented as a finding in other entities that involve fibrosis of the subendocardial and mid myocardial layers (such as post-myocardial infarction scar and dilated cardiomyopathy) (4, 5). The mechanism of fatty infiltration post-MI may be due to ineffective fatty acid metabolism in injured myocytes leading to fat deposition or abnormal differentiation of cells into adipocytes in regions or fibrosis/scar (5, 6). The potential mechanism in this case is unknown.
Blood pool nulling compared to viable myocardium nulling has been shown to improve the detection of ischemic scar. Specifically, it improves visualization of subendocardial hyperenhancement due to improved contrast to noise between the scar and left ventricular blood pool (7, 8). Based on this, acquisition of viable myocardium nulled images would have been reasonably expected to underestimate the subendocardial fibrosis burden, and therefore were not acquired. Patients with LE may have a dramatic response to treatment with imatinib, particularly early in the disease course (9). Near complete reversal has been noted by CMR (10). Contraction and/or regression of the subendocardial fibrotic layer has been postulated as an explanation. Drug therapy with Imatinib may have anti-fibrotic mechanisms acting through inhibition of the PDGF receptor (11). Serial assessment of the subendocardium by CMR as response to therapy has not been explored. Phase sensitive inversion recovery (PSIR) blood pool nulling would provide more accurate identification of subendocardial enhancement in this context. Our patient was being followed by CMR for response to the therapy, with several treatment decisions being linked to CMR findings (for instance, LV thrombus) in the past. Overall, we have highlighted the utility of blood pool nulling and its usefulness in visualization of subendomyocardial fibrosis in our patient with LE.
Click here to view the entire study via CloudCMR
References:
7. Holtackers R. J., et al. Dark-blood late gadolinium enhancement without additional magnetization preparation. J Cardiovasc Magn Reson. 2017; 19(1): 64.
9. Lee S, Choi CU, Kim EJ, Na JO. Regression of biventricular Loeffler’s endocarditis after early treatment, European Heart Journal – Cardiovascular Imaging. May 2017;18(5): 610
10.Faria R. Loeffler’s endocarditis—A case report. Rev Port Cardiol. 2012; 31(6): 445-448
11. Wang L-X, Yang X, Yue Y, Fan T, Hou J,Chen G-X, et al. (2017) Imatinib attenuates cardiac fibrosis by inhibiting platelet-derived growth factor receptors activation in isoproterenol induced model. PLoS ONE. 2017; 12(6): e0178619
Case prepared by:
Dr Rebecca Kozor, BSc(Med) MBBS PhD FRACP FCSANZ
Associate Editor, SCMR Case of the Week
Royal North Shore Hospital and the University of Sydney, Sydney, Australia





