Daniel S Kikuchi, MDa*, Clive A Goulbourne, MDb, & Anurag Sahu, MDb
a Osler Medical Residency, The Johns Hopkins Hospital, Baltimore, MD, USA
b Division of Cardiology, Department of Medicine, Emory University, Atlanta, GA, USA
* Corresponding Author
Clinical History
A 24-year-old male with a history of cardiac tumor status post resection presented to the emergency department with several days of cough productive of bright red blood. He reported that the cough was constant and not associated with chest pain or palpitations. On review of systems, he only endorsed shortness of breath but otherwise denied additional constitutional, cardiovascular, or infectious complaints.
Of note, the patient reported having similar symptoms three months earlier. At that time, he presented to an outside hospital where he was found to have a large obstructive mass in the left atrium (Video 1) and underwent resection. Surgical specimens were sent to pathology, which confirmed the diagnosis of undifferentiated pleomorphic sarcoma. The patient was discharged home in stable condition with follow up at our cancer institute.
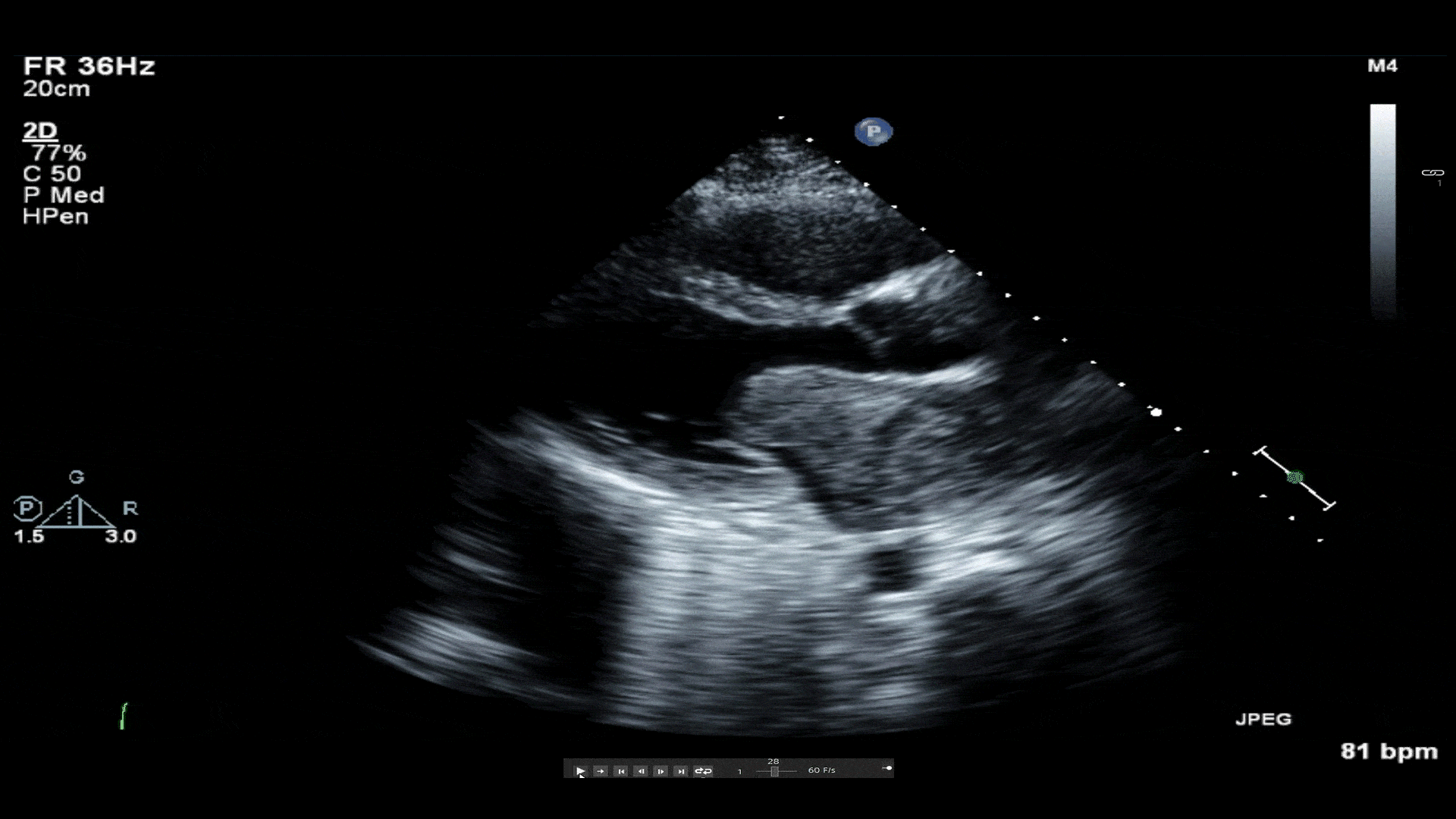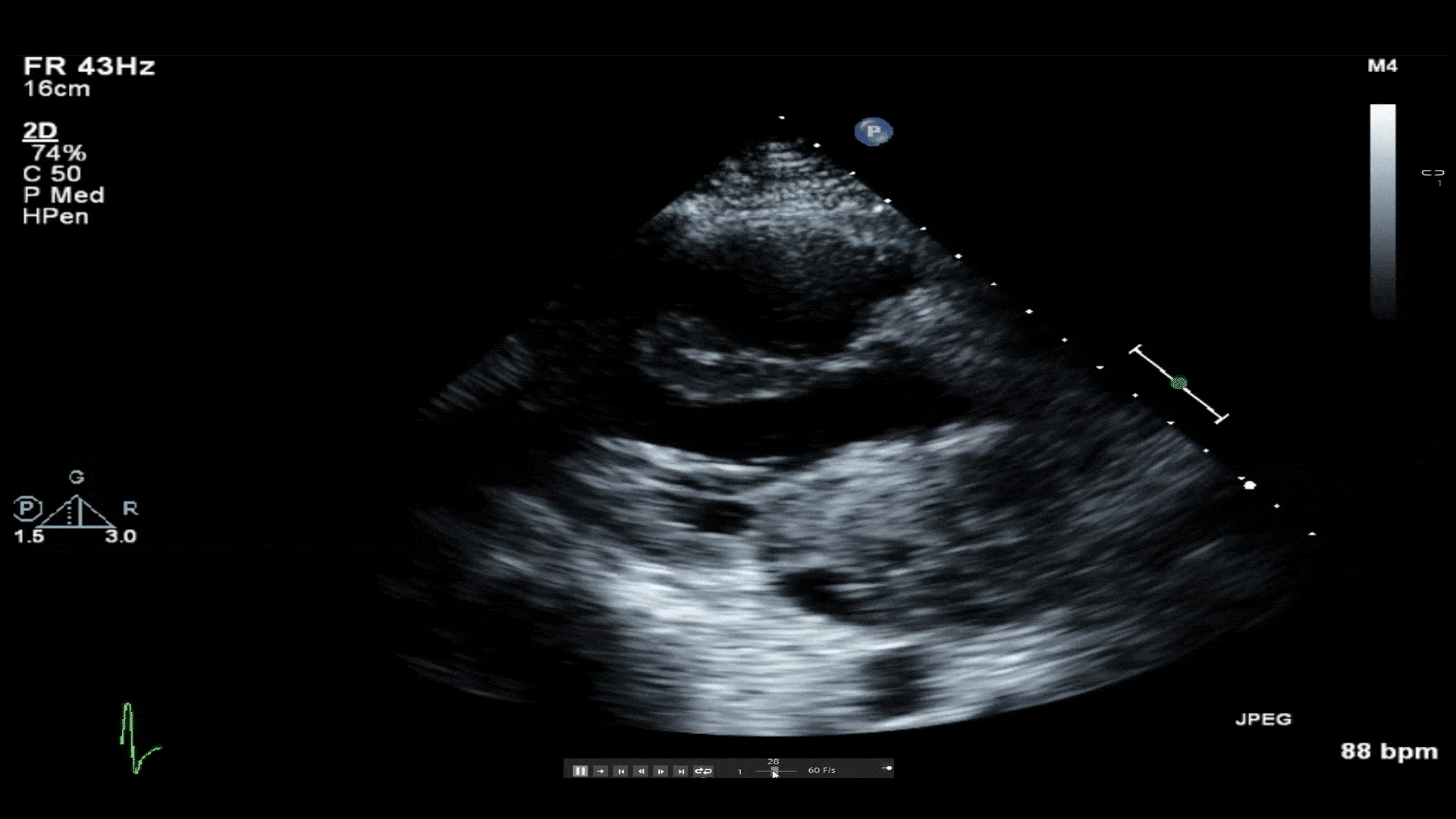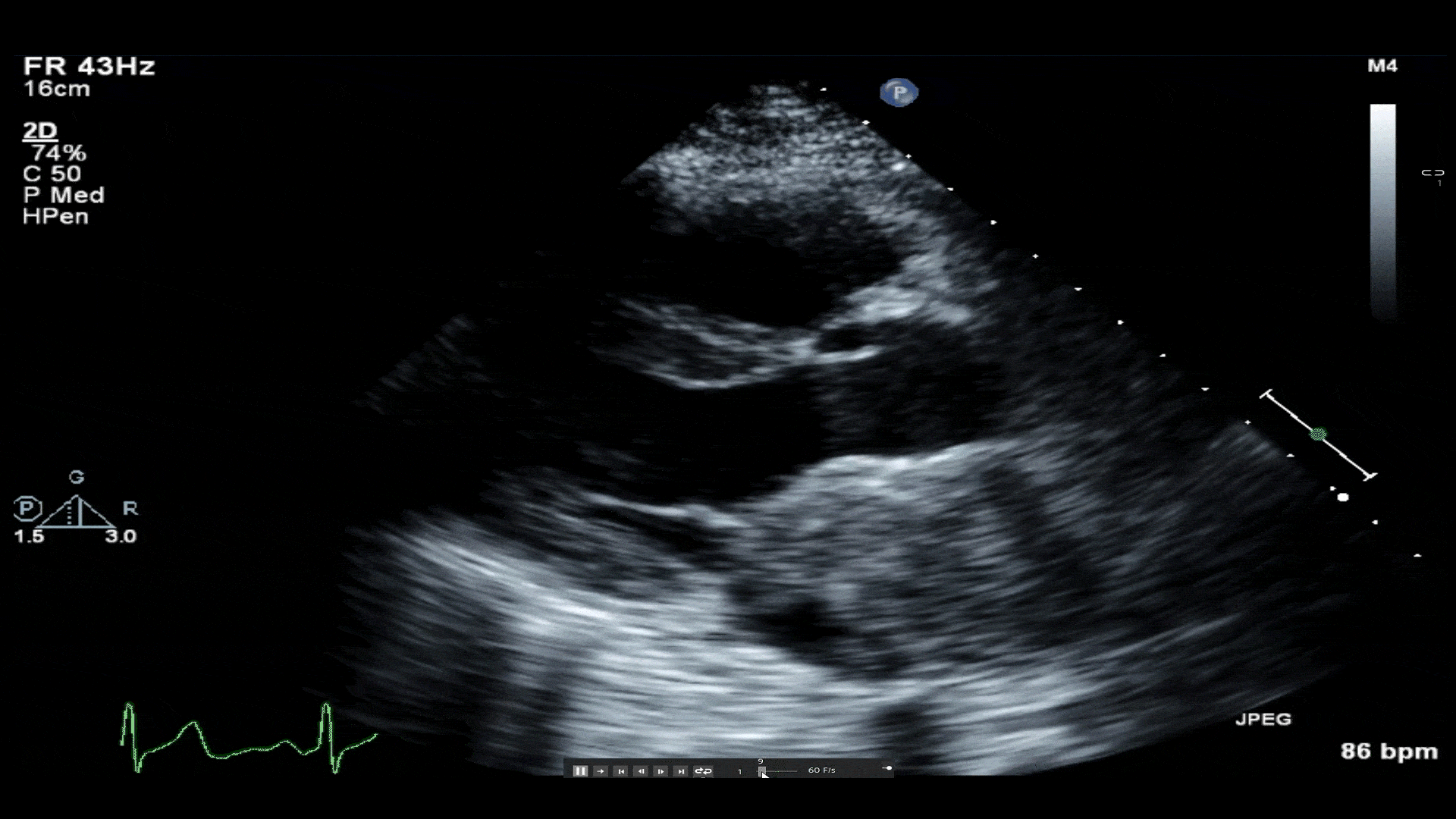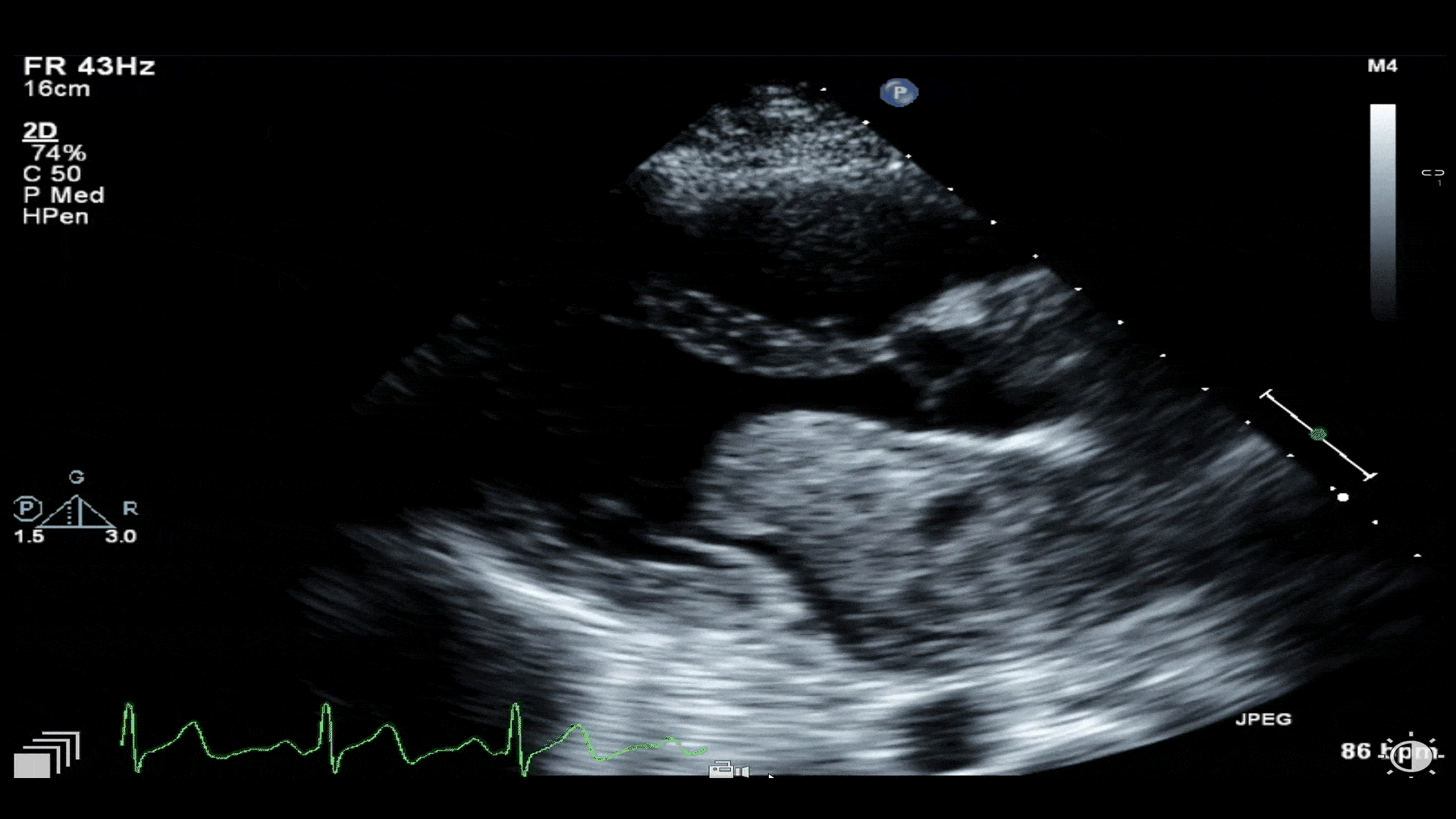
Video 1: Transthoracic echocardiogram revealing a large mass in left atrium protruding through the mitral valve. A small hypoechoic area of necrosis is seen within the mass.
Approximately one month after resection, routine surveillance by TTE (Video 2) revealed an irregularity in the posterior left atrium.
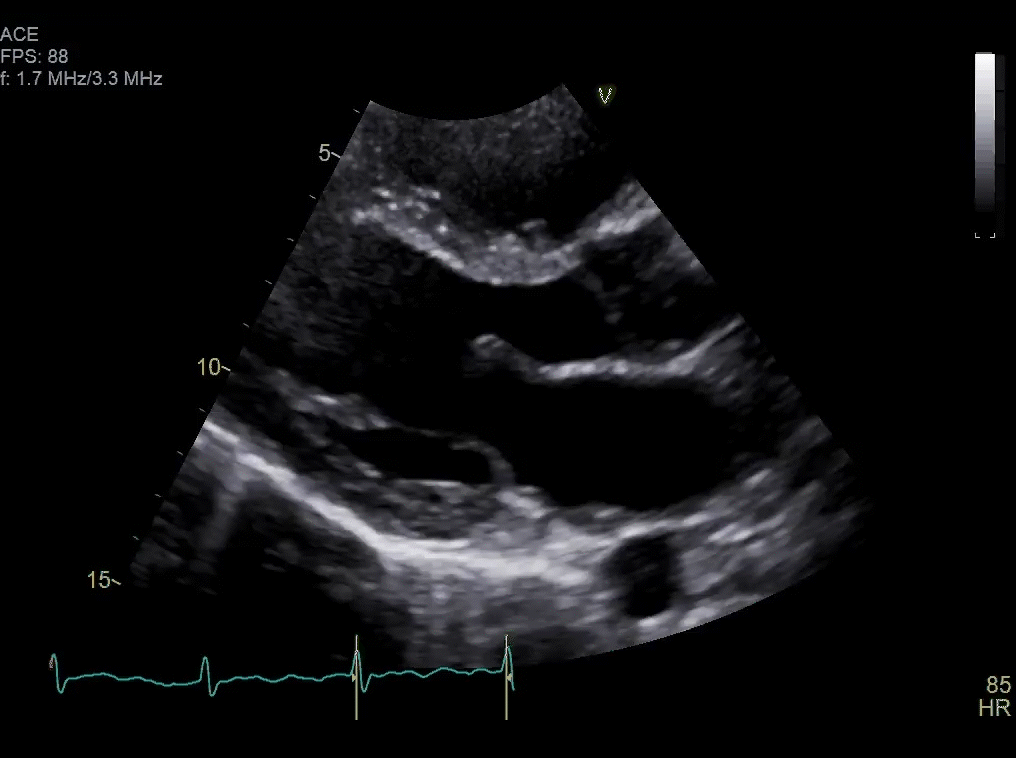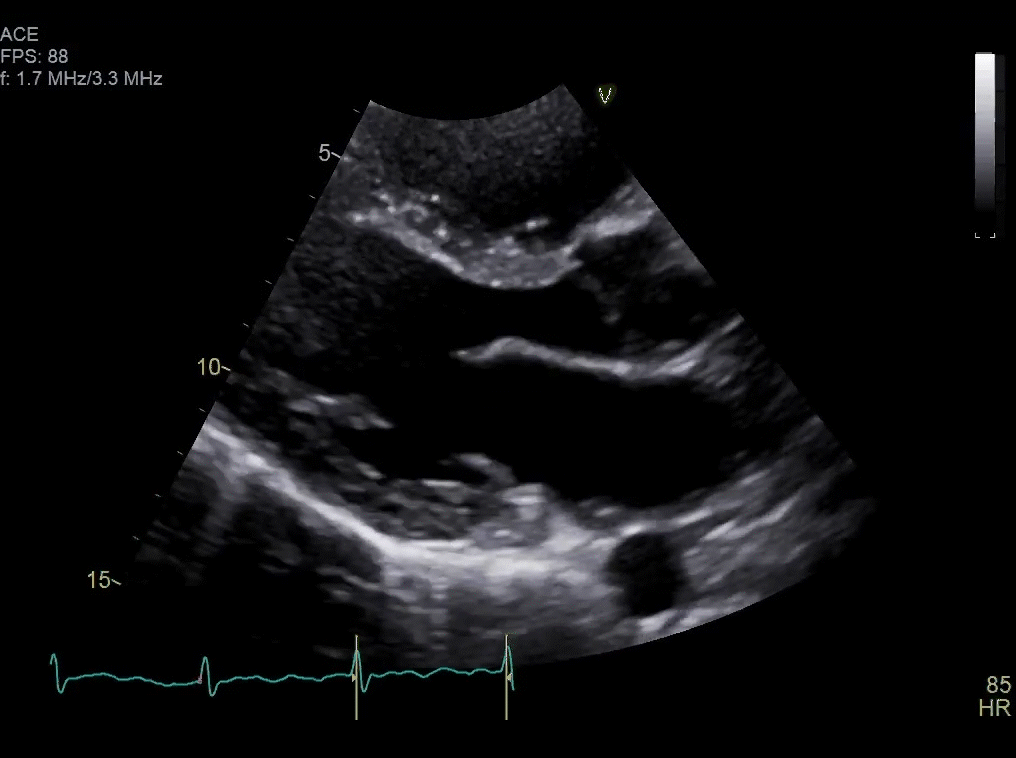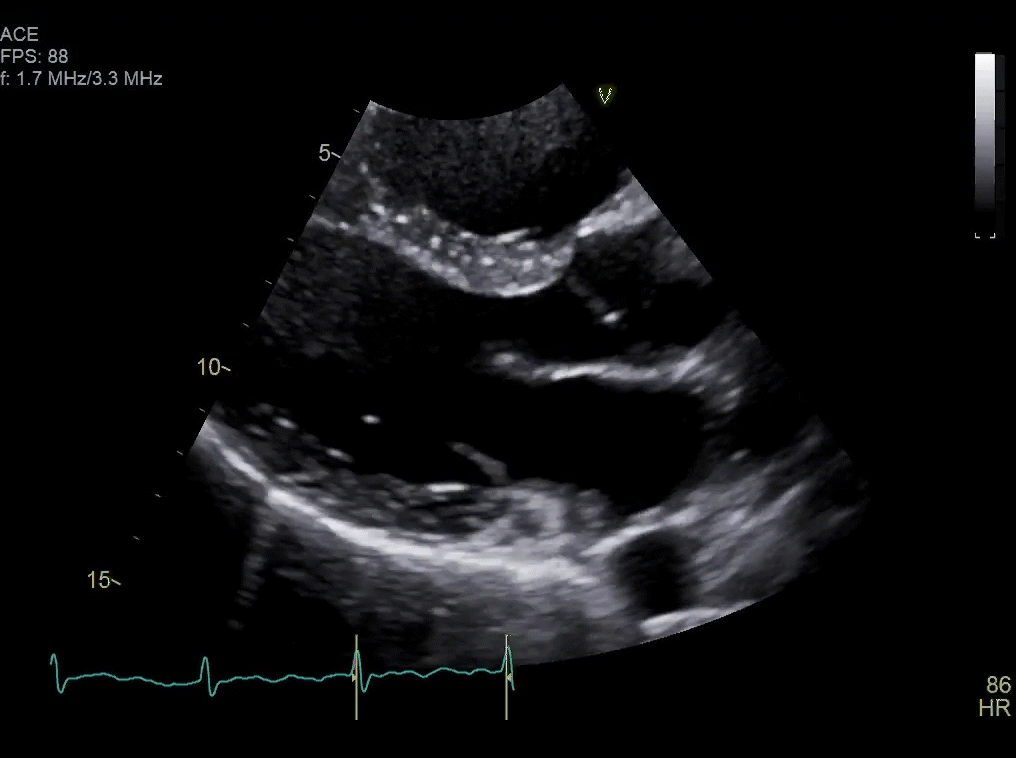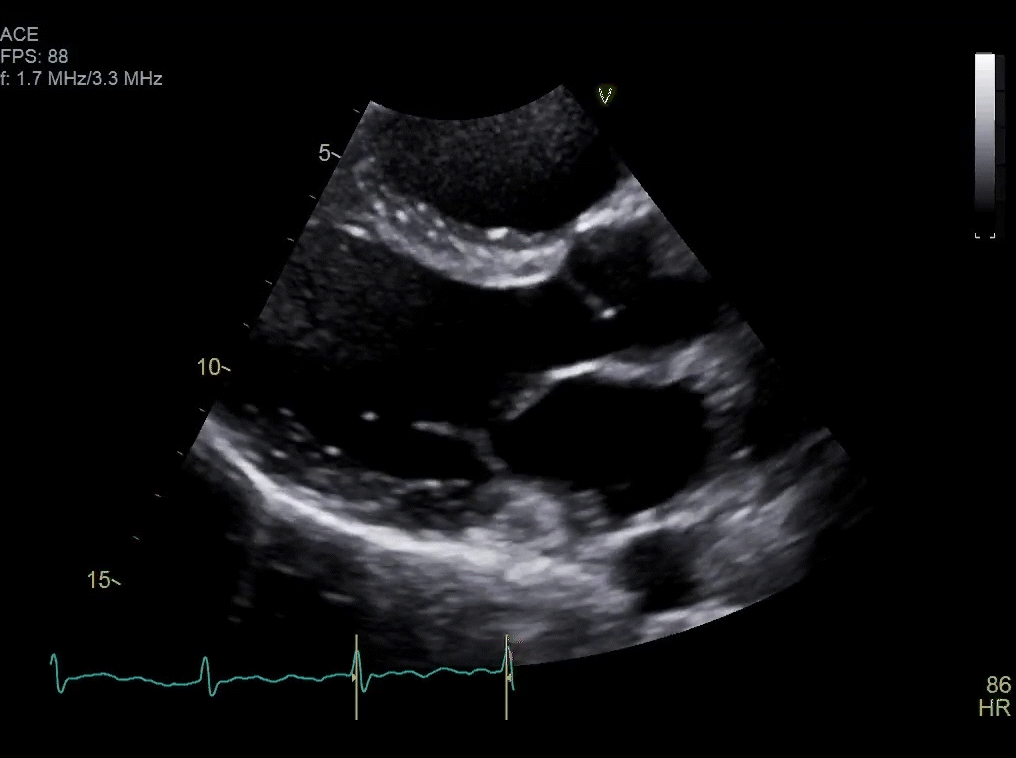
Video 2: TTE post-surgical resection of left atrial mass. Note small area of irregularity in the posterior left atrium which may represent post-surgical changes or residual mass.
Further investigation with PET CT scan of the entire body and CMR was recommended by our institutional tumor board. PET CT scan (Image 1) revealed a low level of FDG uptake in the left atrium as well as intensely FDG avid lytic lesions in the left pelvis and interdigitating edema with photopenia in the right frontoparietal lobes, concerning for metastatic disease.
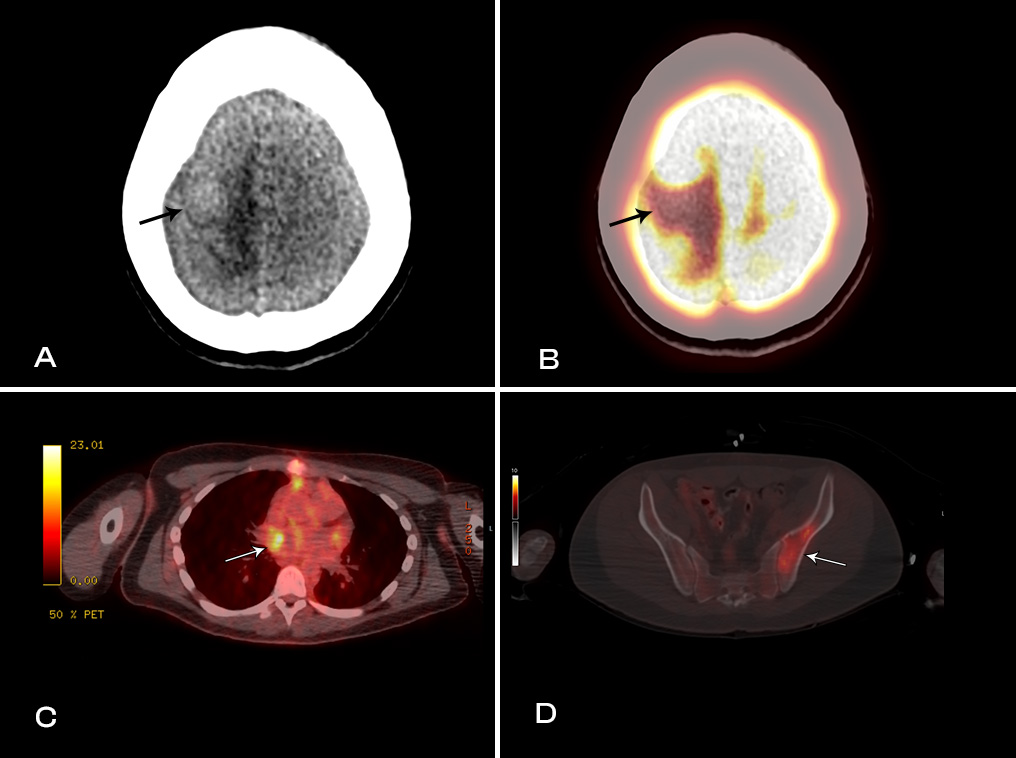
Image 1: PET-CT images showing metastatic lesions with interdigitating edema (A) with photopenia in the high right fronto-temporal lobes (B). Low level FDG uptake in the left atrium (C) and intensely FDG avid lesions involving left pelvis (D).
Subsequently, MRI of the brain with contrast (Video 3) confirmed the presence of two solid enhancing intracranial masses.
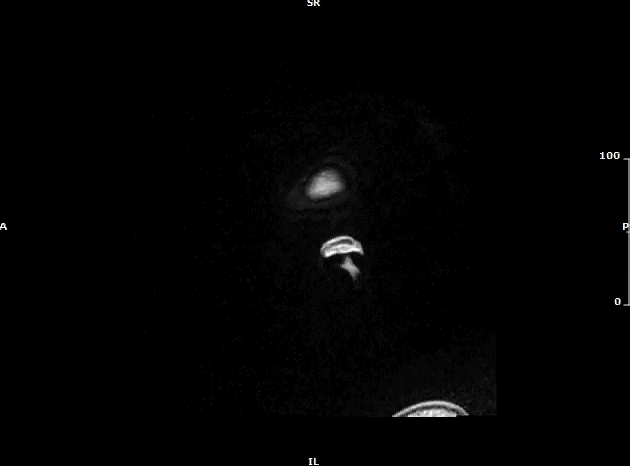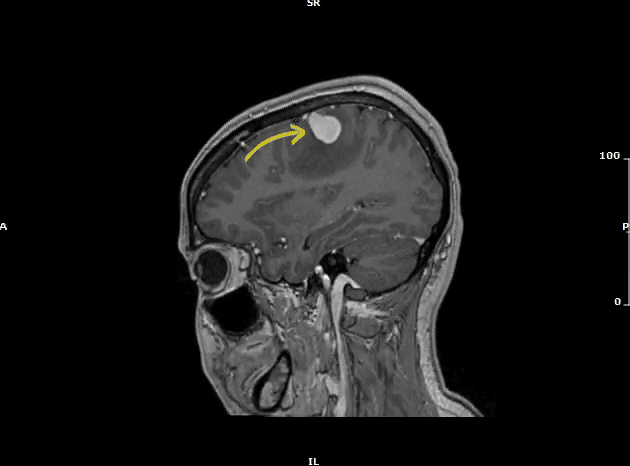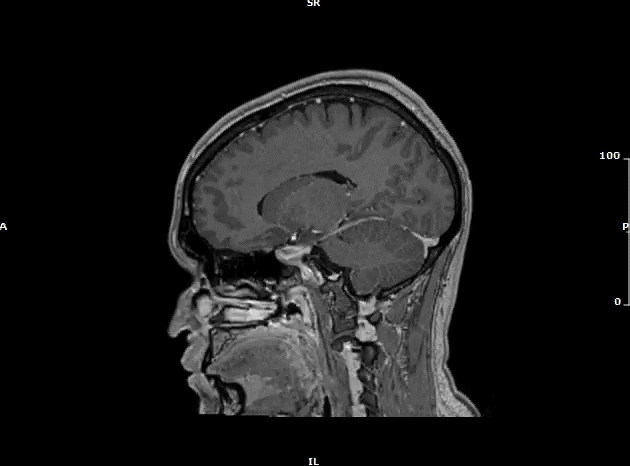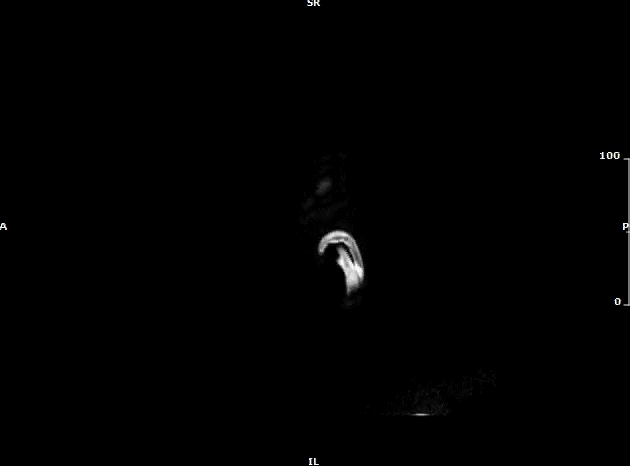
Video 3: MRI brain (sagittal view) showing two enhancing intracranial masses (yellow arrows) due to metastasis.
In the emergency department, vital signs were notable for tachycardia to the 120’s, physical exam was unremarkable, and laboratory studies revealed an elevated white blood cell count. CT scan with IV contrast (Image 2) was significant for a large hypoenhancing filling defect in the left atrium extending into the right upper and middle pulmonary veins. Further evaluation of the cardiac mass with TTE (Video 4) revealed a large tumor occupying two-thirds of the left atrial volume.
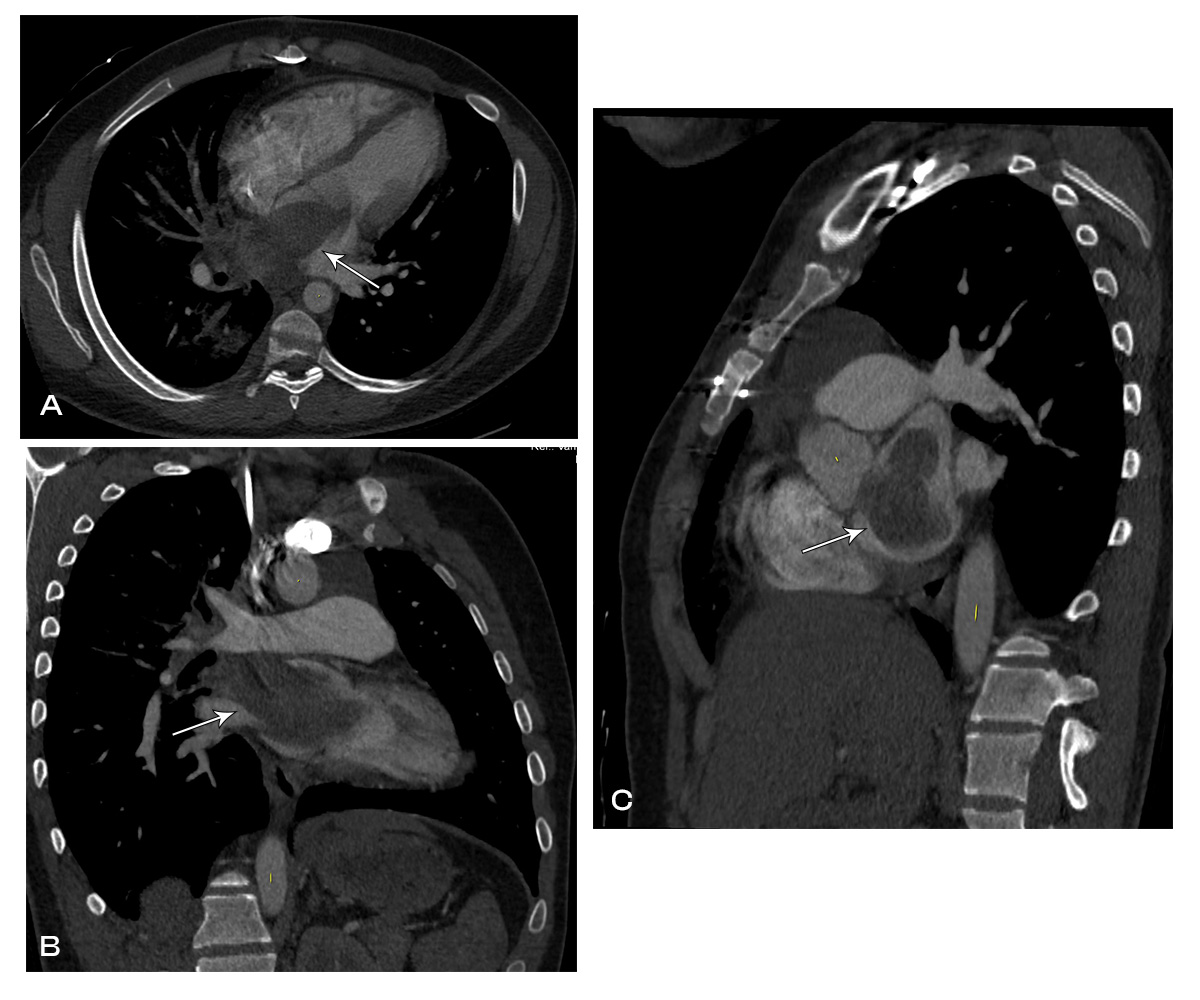
Image 2: CT chest with contrast showing left atrial mass extending into the right upper and right middle pulmonary veins.
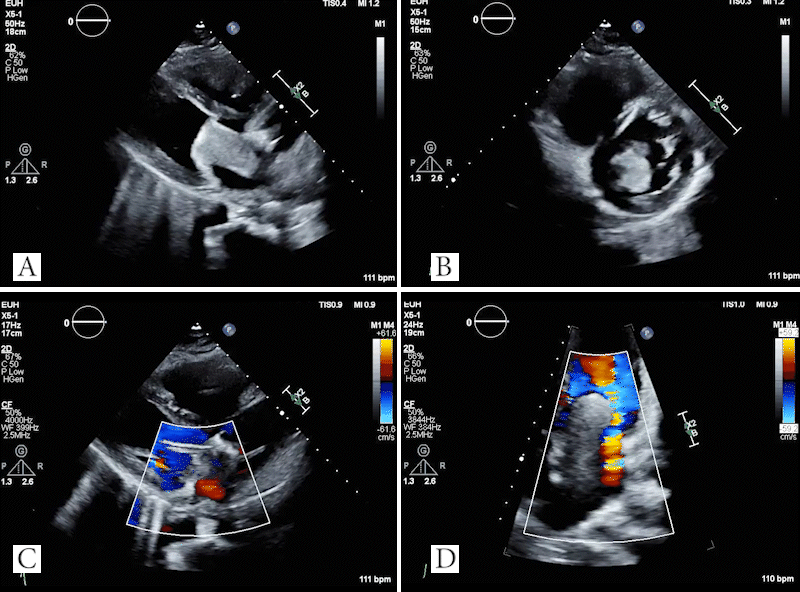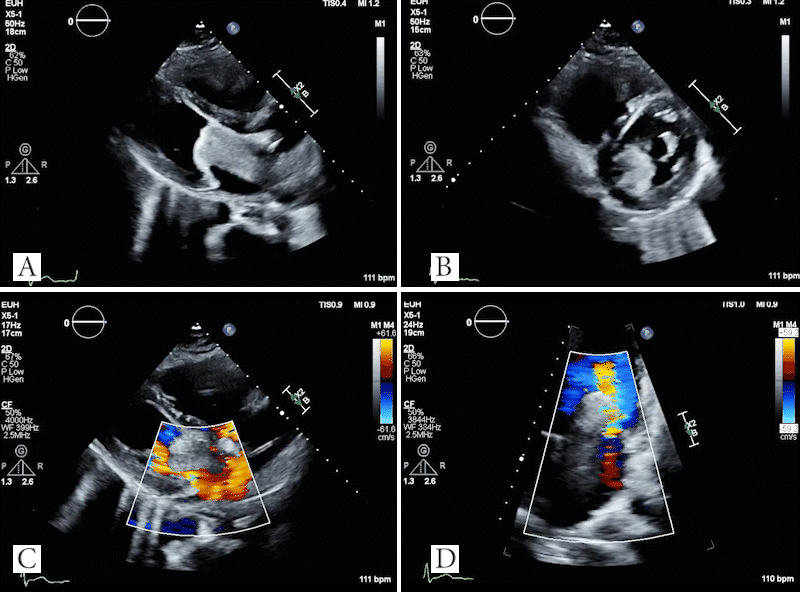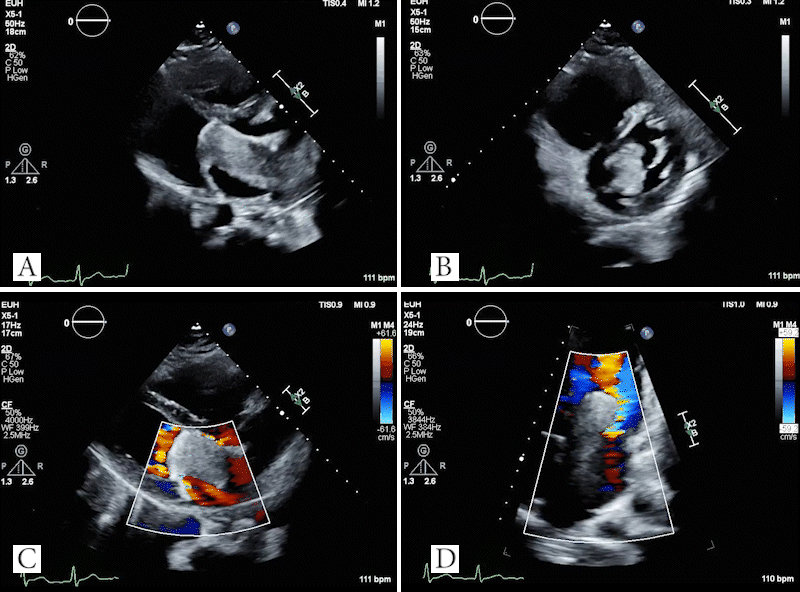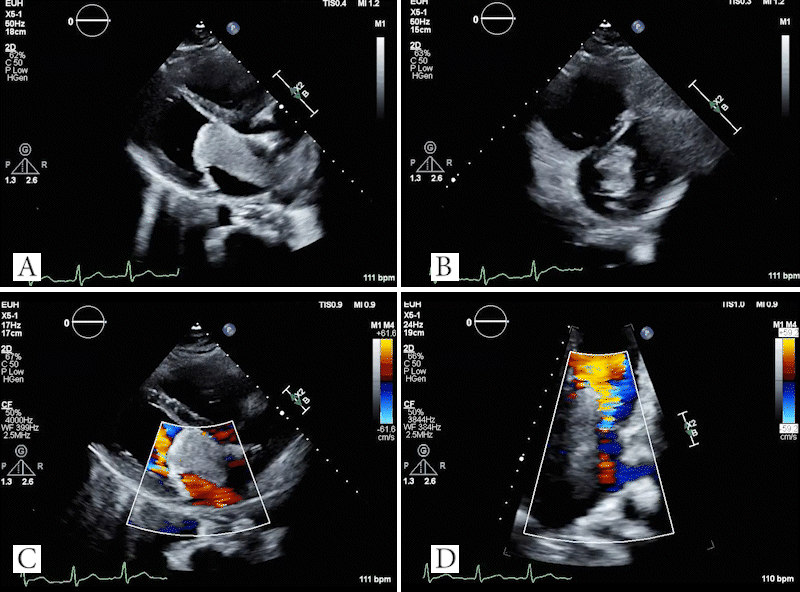
Video 4: TTE showing large mass in left atrium which obstructs the mitral valve in the parasternal long axis (A) and parasternal short axis (B) views. Doppler images showing evidence of flow acceleration due to mitral valve obstruction (C, D).
The patient was admitted to the Hematology and Oncology Service for further evaluation and management.
CMR Findings
CMR was performed prior to admission approximately two weeks after the transthoracic echocardiogram revealed an irregularity in the posterior left atrium but no distinct recurrent mass. The patient was asymptomatic at the time of this initial CMR. This study delineated a large, bilobed mass with vascular components in the left atrium extending from the right upper pulmonary vein. On CMR, the larger lobe measured 3.1 x 2.8 x 4.2 cm and the smaller lobe measured 2 x 3.1 x 2 cm (Video 5). Tissue characterization revealed the mass was isointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and diffusely enhancing on delayed gadolinium imaging (Image 3, Image 4). The mass appeared avascular on first pass perfusion imaging (Video 6).
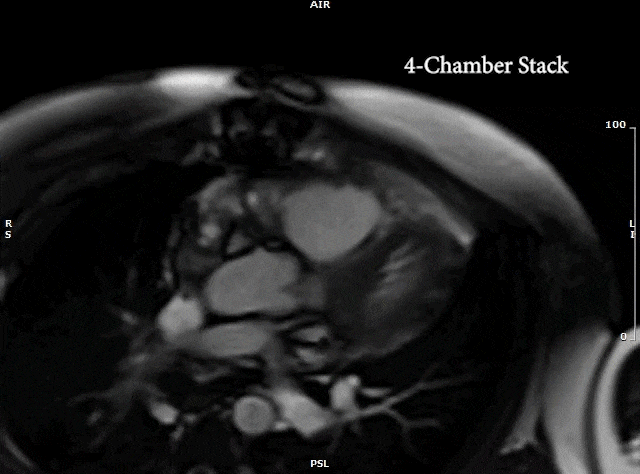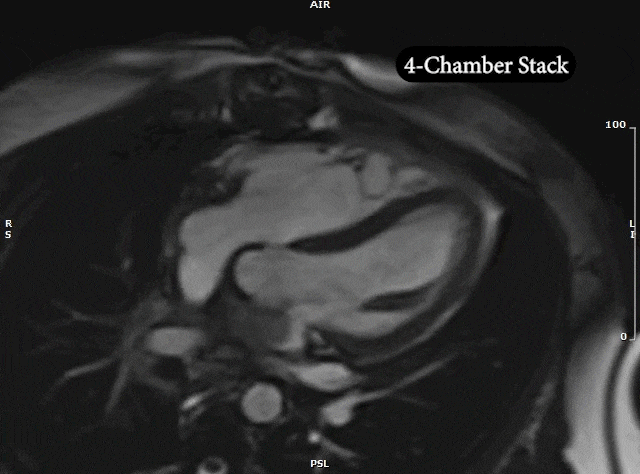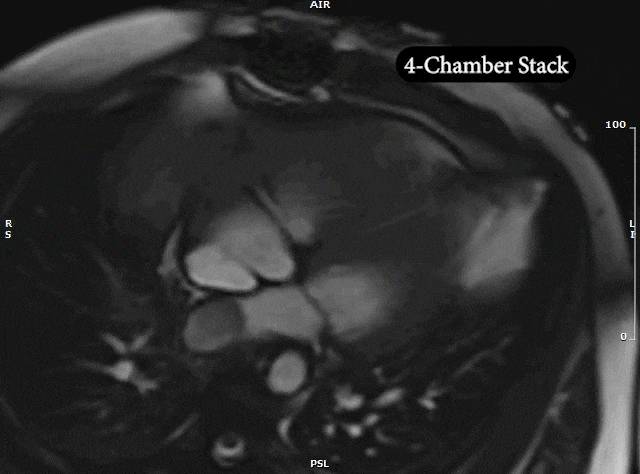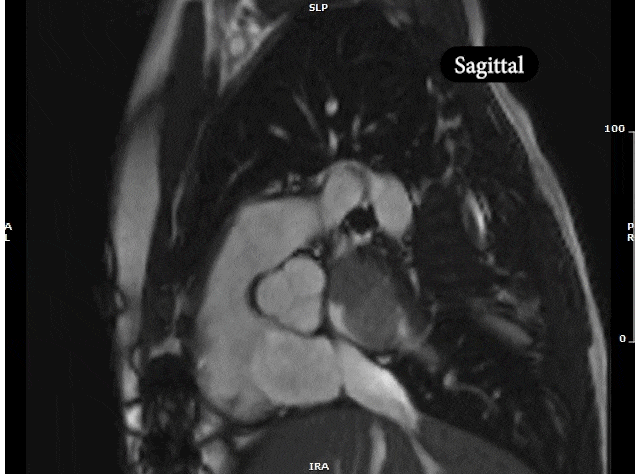
Video 5: MRI cardiac (SSFP) cines (4 chamber, 2 chamber & sagittal views) showing recurrence of large mass in the left atrium extending into the right pulmonary veins.

Image 3: MRI cardiac T1-weighted dark blood sequences showing hypointense left atrial mass extending into the right pulmonary veins (A, D). The mass was hyperintense on T2-weighted dark blood (B, E) and T2-weighted fat saturation (C, F) sequences.
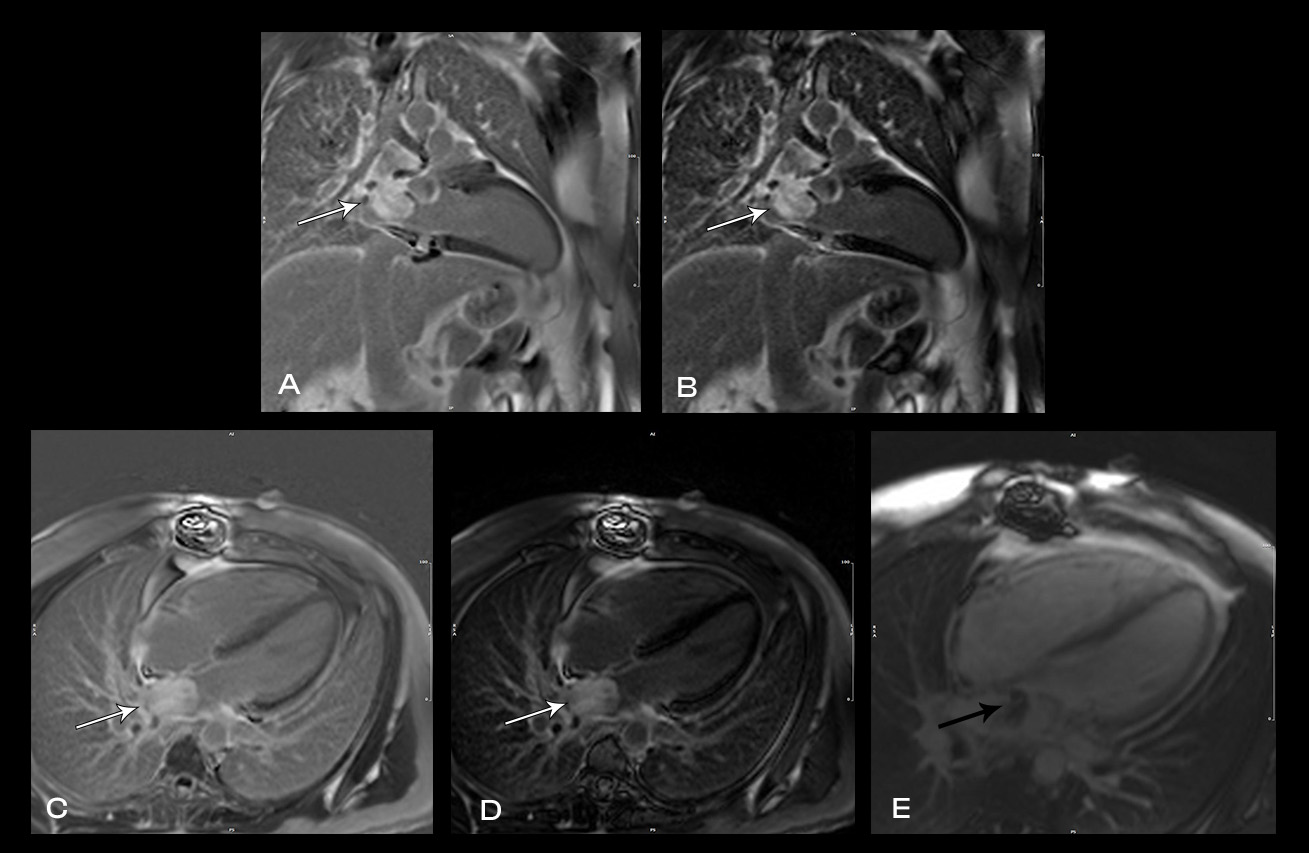
Image 4: MRI cardiac showing heterogenous late gadolinium enhancement (LGE) of the left atrial mass on magnitude (A, C) and phase sensitive inversion recovery (PSIR) sequences (B, D). Ti-600 sequences suggested evidence of some thrombus adherent to the mass (E).
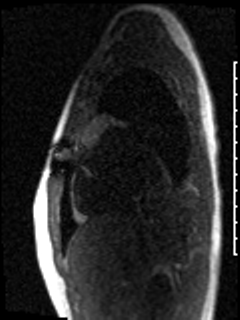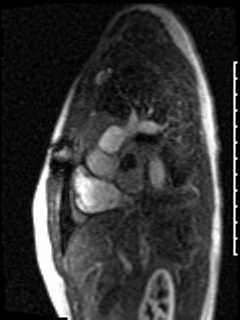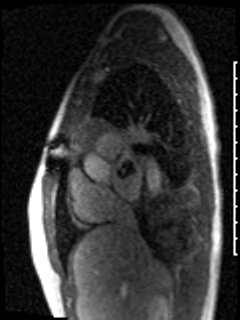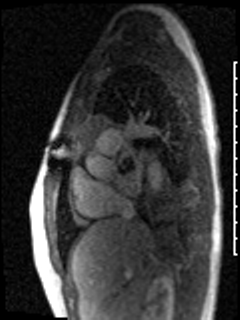
Video 6: The left atrial mass appears avascular on first pass perfusion imaging.
Following urgent radiation therapy and initiation of chemotherapy, CMR was repeated during the patient’s hospitalization (Video 7). The mass now measured 8.6 x 3.6 x 4.2 cm. On this CMR, the cardiac mass was noted to prolapse though the mitral valve during diastole resulting in severe mitral valve obstruction and mild left ventricular outflow tract obstruction.
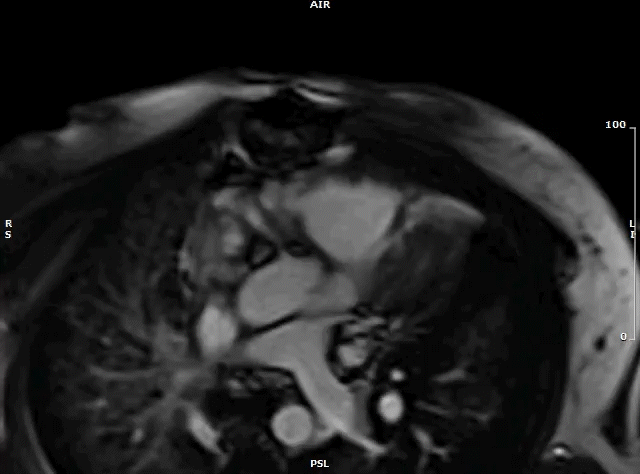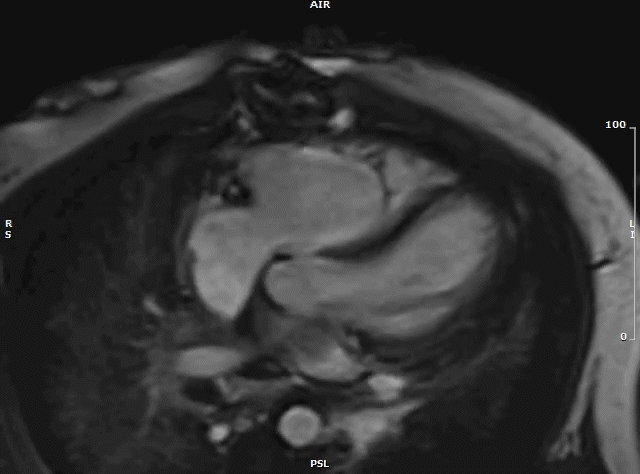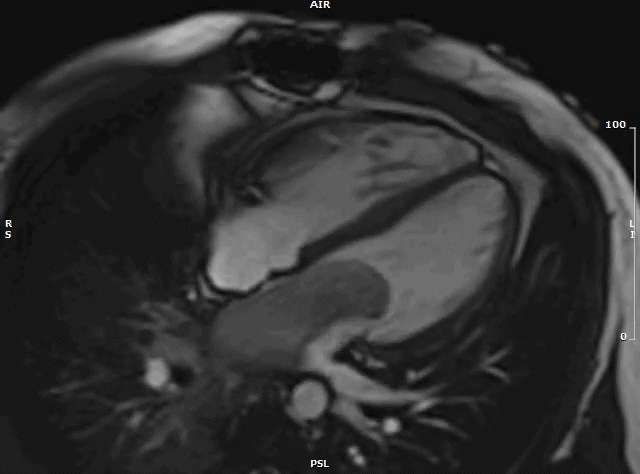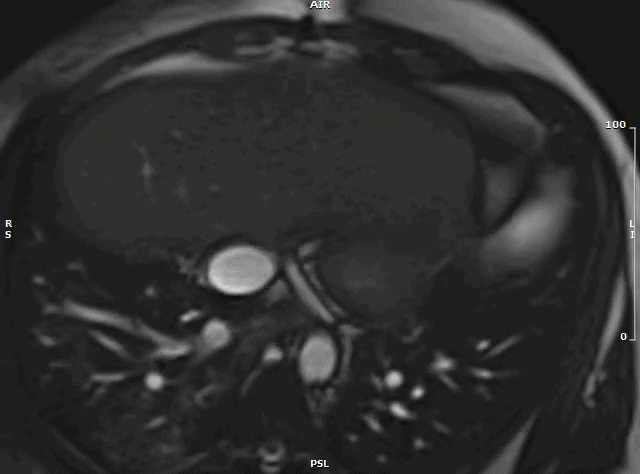
Video 7: MRI cardiac (SSFP) cines (4 chamber stack) showing the large left atrial mass prolapsing across the mitral valve.
Conclusion
Both CMRs revealed a large, obstructive left atrial mass in a patient with a known history of undifferentiated pleomorphic sarcoma. Recurrence of the sarcoma, with rapid growth over a three-week period likely precipitated the patient’s presentation. His hospital course was complicated by acute hypoxic respiratory failure secondary to obstructive mass effect resulting in pulmonary edema and post-obstructive pneumonia. The patient was not a surgical candidate and underwent urgent palliative chemotherapy and radiation therapy. One month after being admitted he was discharged home in stable condition. He continues palliative chemotherapy as an outpatient and his symptoms are well controlled.
Perspective
Primary cardiac tumors are rare with a prevalence between 0.002% and 0.03% (1). Among these tumors, approximately a quarter are malignant, with sarcomas being the most common (2). Undifferentiated pleomorphic sarcoma, also referred to as malignant fibrous histiocytoma and undifferentiated sarcoma, account for one third of cardiac sarcomas (3). Most undifferentiated pleomorphic sarcomas of the heart typically originate in the left atrium (4), as in our case, but are locally aggressive and prone to metastasize. As a result, outcomes are poor (5-7). The standard of care involves resection with adjuvant chemotherapy and/or radiation therapy (6). Complete resection followed by adjuvant medical therapy offers the best outcomes (8), however, clear surgical margins are difficult to obtain and recurrence remains a challenge (9-11). Recurrence in our patient is noteworthy for the rapid rate of re-growth resulting in clinically significant obstruction during the two-week period between the echocardiogram and CMR.
The utility of CMR in the work-up of cardiac masses is well established (12). In particular, the unique ability to discriminate differences in tissue densities and the resulting signal patterns on T1- and T2-weighted techniques can often help narrow the differential. Undifferentiated sarcomas of the heart are classically isointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and heterogenous on delayed gadolinium imaging (12, 13). Consistent with these characteristics, the tumor in our patient was isointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and diffusely enhancing on delayed gadolinium imaging. Thus, in addition to the patient history, the CMR with signal pattern consistent with sarcoma as well as the rapid interval increase in size assessed by CMR all supported the diagnosis of recurrent undifferentiated pleomorphic sarcoma complicated by obstructive mass effect.
View the entire CMR cases on Cloud CMR:
References
1. Gupta R, Meghrajani V, Desai R, Gupta N. Primary Malignant Cardiac Tumors: A Rare Disease With an Adventurous Journey. Journal of the American Heart Association. 2020;9(10):e016032.
2. Antwi‐Amoabeng D, Meghji Z, Thakkar S, Ulanja MB, Taha M, Adalja D, et al. Survival Differences in Men and Women With Primary Malignant Cardiac Tumor: An Analysis Using the Surveillance, Epidemiology and End Results (SEER) Database From 1973 to 2015. Journal of the American Heart Association. 2020;9(10):e014846.
3. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli LG. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-9.
4. Okamoto K, Kato S, Katsuki S, Wada Y, Toyozumi Y, Morimatsu M, et al. Malignant fibrous histiocytoma of the heart: case report and review of 46 cases in the literature. Intern Med. 2001;40(12):1222-6.
5. Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, et al. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Ann Surg Oncol. 2009;16(12):3358-65.
6. Randhawa JS, Budd GT, Randhawa M, Ahluwalia M, Jia X, Daw H, et al. Primary Cardiac Sarcoma: 25-Year Cleveland Clinic Experience. Am J Clin Oncol. 2016;39(6):593-9.
7. Siontis BL, Zhao L, Leja M, McHugh JB, Shango MM, Baker LH, et al. Primary Cardiac Sarcoma: A Rare, Aggressive Malignancy with a High Propensity for Brain Metastases. Sarcoma. 2019;2019:1960593.
8. Ramlawi B, Al-jabbari O, Blau LN, Davies MG, Bruckner BA, Blackmon SH, et al. Autotransplantation for the Resection of Complex Left Heart Tumors. The Annals of Thoracic Surgery. 2014;98(3):863-8.
9. Gabelman C, Al-Sadir J, Lamberti J, Fozzard HA, Laufer E, Replogle RL, et al. Surgical treatment of recurrent primary malignant tumor of the left atrium. J Thorac Cardiovasc Surg. 1979;77(6):914-21.
10. Okita Y, Miki S, Ueda Y, Tahata T, Sakai T, Matsuyama K. Recurrent malignant fibrous histiocytoma of the left atrium with extracardiac extension. Am Heart J. 1994;127(6):1624-8.
11. Sun J, Liu R, Wang W, Sun M, Wang L, Wang X, et al. Primary cardiac malignant fibrous histiocytoma with vulvar metastases: A case report. Oncol Lett. 2015;10(5):3153-6.
12. Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268(1):26-43.
13. Munin MA, Goerner MS, Raggio I, Wisner J, Tettamanzi A, Godia J, et al. A Rare Cause of Dyspnea: Undifferentiated Pleomorphic Sarcoma in the Left Atrium2017.
Case Prepared By:
Robert D. Tunks, MD, MHS
Associate Editor, Cases of SCMR
Penn State Health, Milton S. Hershey Medical Center
Hershey, PA USA







