Case from: Sylvia SM Chen
Institute: Flinders Medical Centre, Adelaide, Australia
Clinical history: A 51 year old male was referred for a CMR study. He had repair of Tetralogy of Fallot in childhood, and an aortic valve replacement about 9 years ago. The purpose for CMR study was for assessment of pulmonary arteries and pulmonary regurgitation (PR). No other details were available from the referral letter or the patient, who otherwise seemed relatively well and apparently symptom free.
CMR findings:
The right ventricle (RV) was dilated (Figure 1a-b) with an indexed RVEDV of 198ml/m2, RVESV 130ml/m2, and reduced overall RVEF 34%, partly due to the moderately large akinetic region in the RV outflow tract representing the patch inserted during surgical repair (Figure 1a). Importantly also, the left ventricle (LV) was dilated (Figure 1b), with an indexed LVEDV of 167ml/m2, LVESV 109ml/m2, and reduced LVEF of 35% (no residual ventricular septal defect found). There was almost free PR (Figure c-d) with a regurgitation fraction of 37% by velocity mapping.
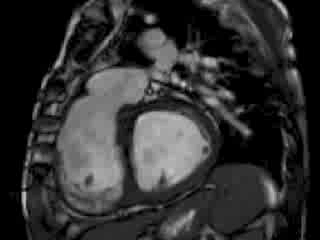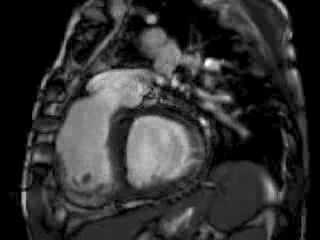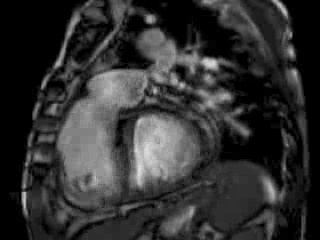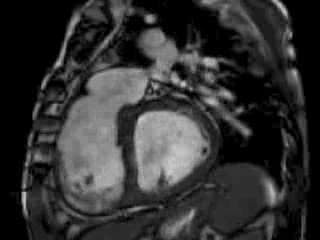

Fig. 1a & Fig 1.b
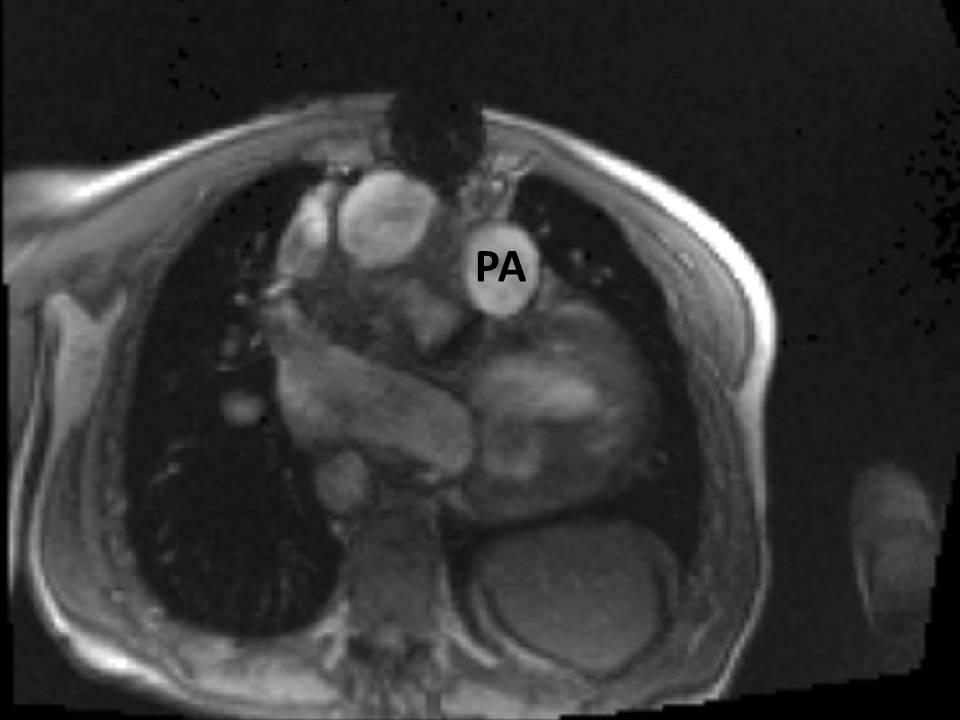
Fig. 1a & Fig 1.b
Figure 1: a. Dilated RVOT with PR, and moderately large akinetic thinned region in the anterior wall of the RVOT; b. Dilated RV and LV with flattened septum indicative of RV volume overload; c-d. Magnitude (c) image for corresponding flow velocity map (d) in the transverse orientation through the pulmonary artery (PA), forward flow in white, and reverse flow (PR) in black.
The mechanical aortic valve replacement was functioning well, but there was a large chronic haematoma surrounding the grafted aortic root and ascending aorta (Figure 2a-c). No dissection flap or flow into the region was seen.
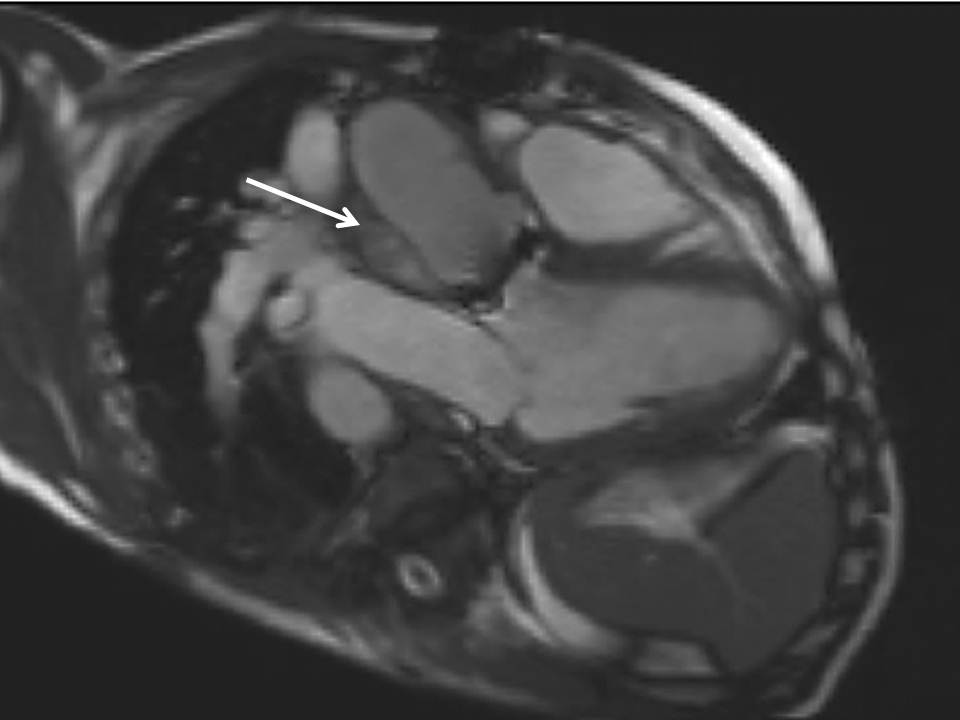
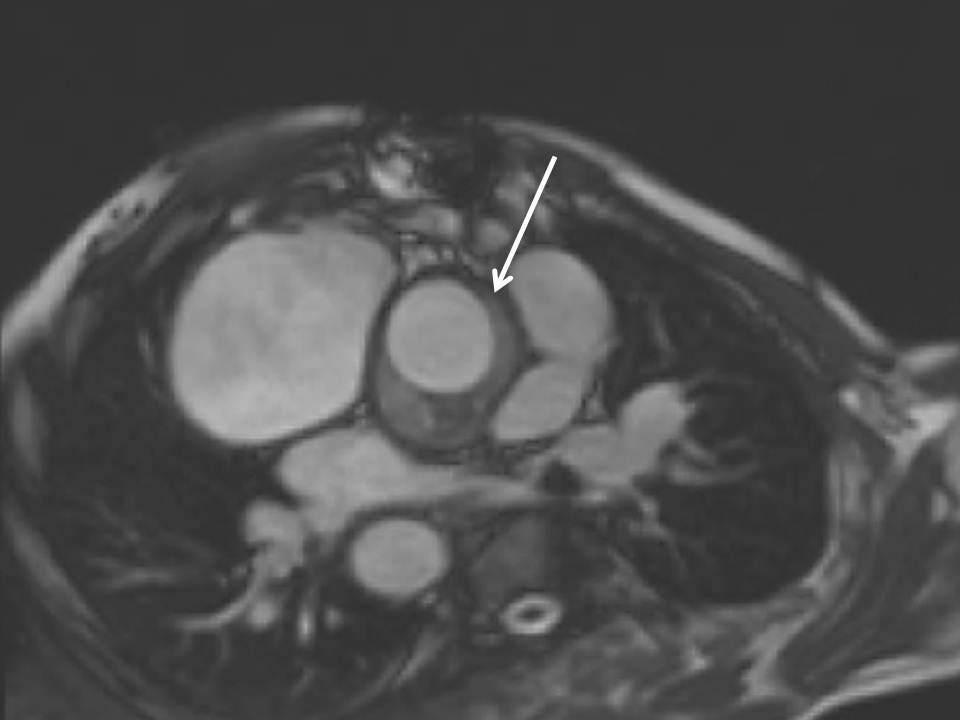
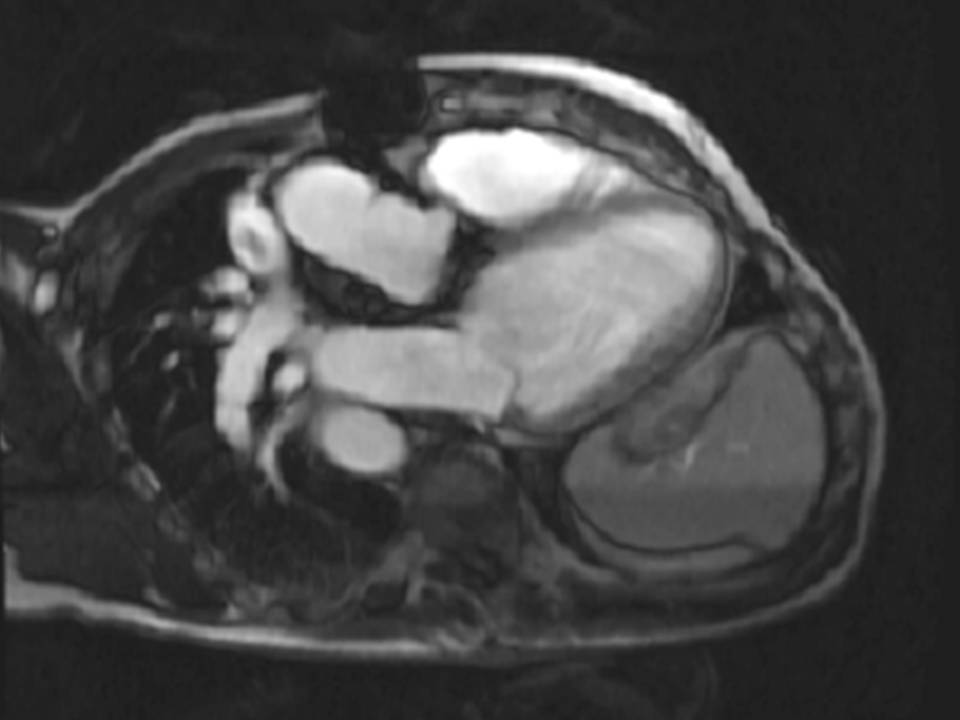
Fig. 2a, Fig. 2b, and Fig. 2c
Figure 2: Haematoma surrounding the aortic root in the LVOT (a) and transverse (b) orientations respectively; and (c) seen early after gadolinium administration, as the black region around the aortic root without contrast uptake.
The origin of the left pulmonary artery (LPA) was distorted and angulated but did not appear significantly stenosed (Figure 3a-b). No stenosis in the right pulmonary artery (RPA, Figure 3c). Flow analysis however, showed reduced flow (19.3ml/beat) in the LPA compared to the RPA (63.3ml/beat), and interestingly, there was flow reversal in late diastole in the LPA (Figure 4) suggesting flow into the LPA from a collateral vessel. Reduced flow in the LPA was surprising, as the LPA did not appear to have significant stenosis on cine imaging. Closer reinspection of the available images showed a vessel passing behind the RPA which may be a collateral vessel (Figure 3c).
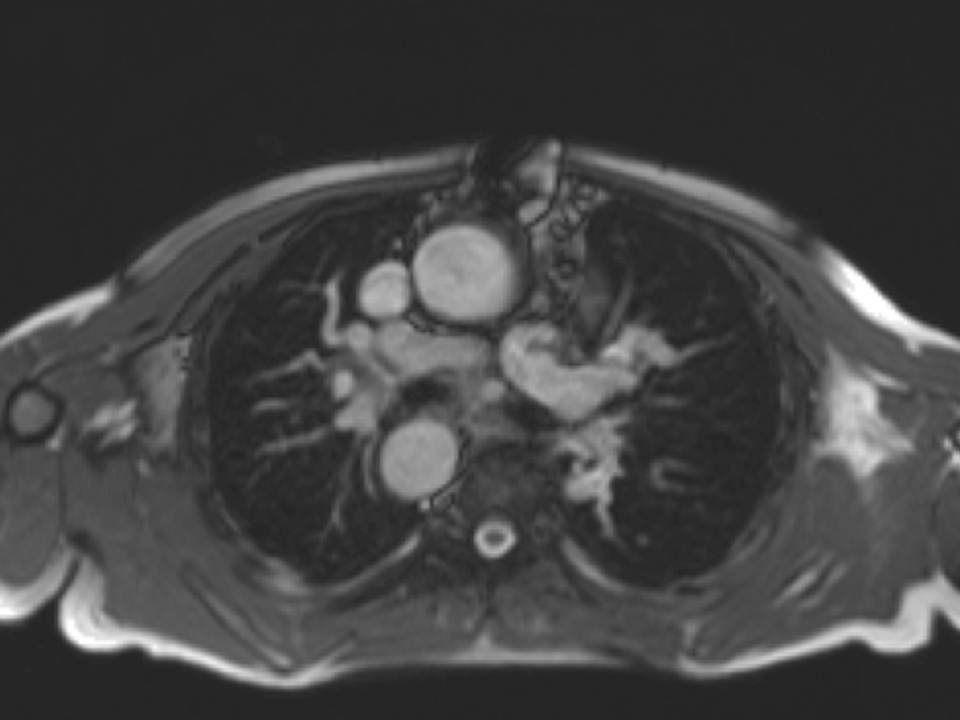
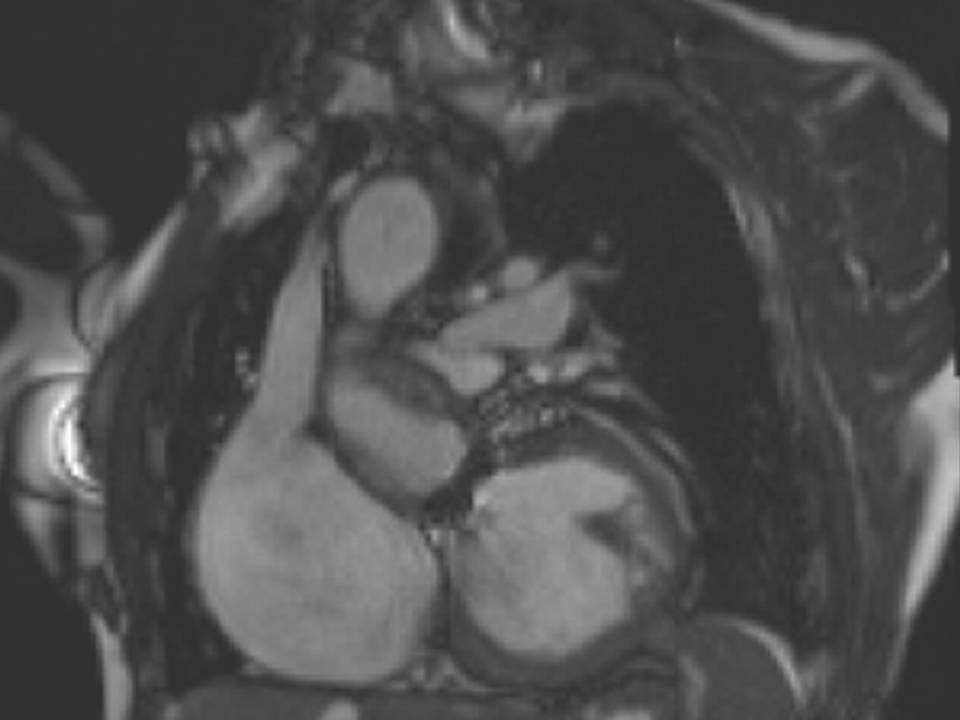
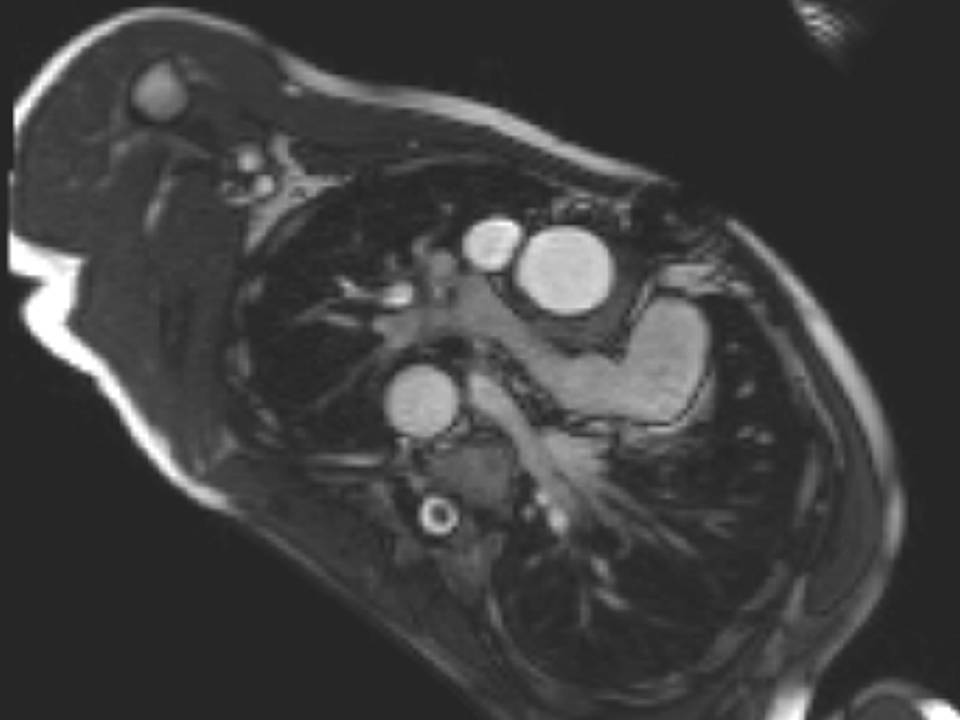
Fig. 3a, Fig. 3b, and Fig. 3c
Figure 3: a. Distorted LPA origin; b. Bifurcation of the pulmonary branch arteries without significant stenosis; c. RPA, and a collateral vessel running posteriorly between the RPA and the spine.
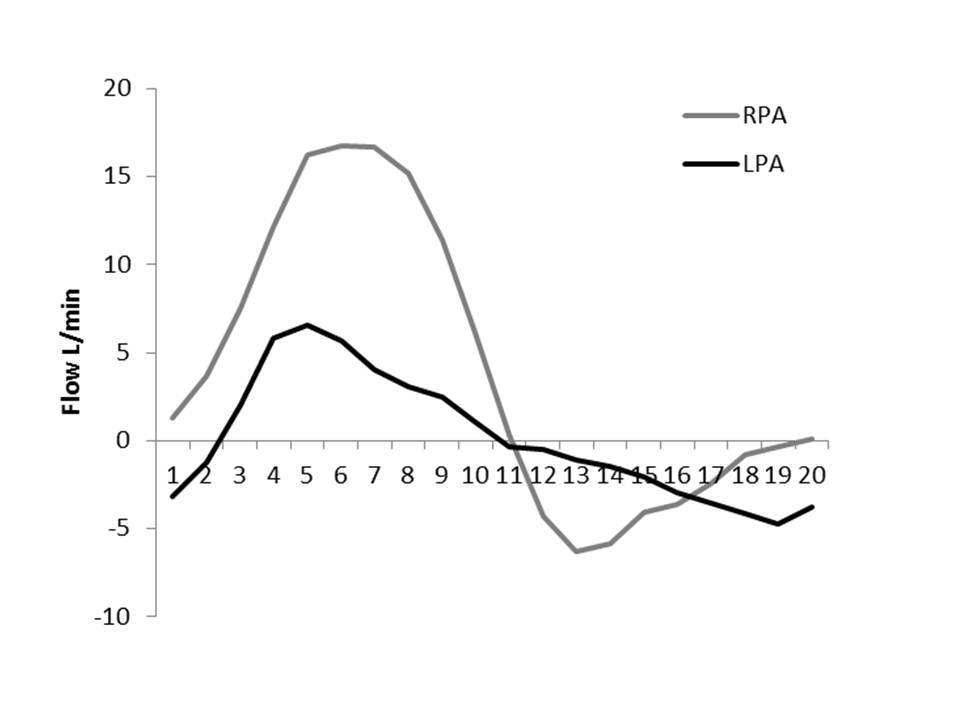
Figure 4: RPA and LPA flow curves.
Contrast enhanced magnetic resonance angiography (MRA) was then performed to look for collateral vessels (Figure 5). This showed a collateral vessel from the distal end of the lower lobe branch of the RPA, and traversing towards the left lung, along the inferior aspect of both bronchi and supplying the left lower lobe. There was also a narrow connection between this anomalous vessel and the proximal LPA (Figure 6a-b). This connection is relatively small, and explains late diastolic flow reversal (Figure 4). The LPA on MRA, predominantly supplies the left upper lobe, and the collateral vessel the left lower lobe. No other collaterals including major aortopulmonary collaterals were seen.

Figure 4: RPA and LPA flow curves.
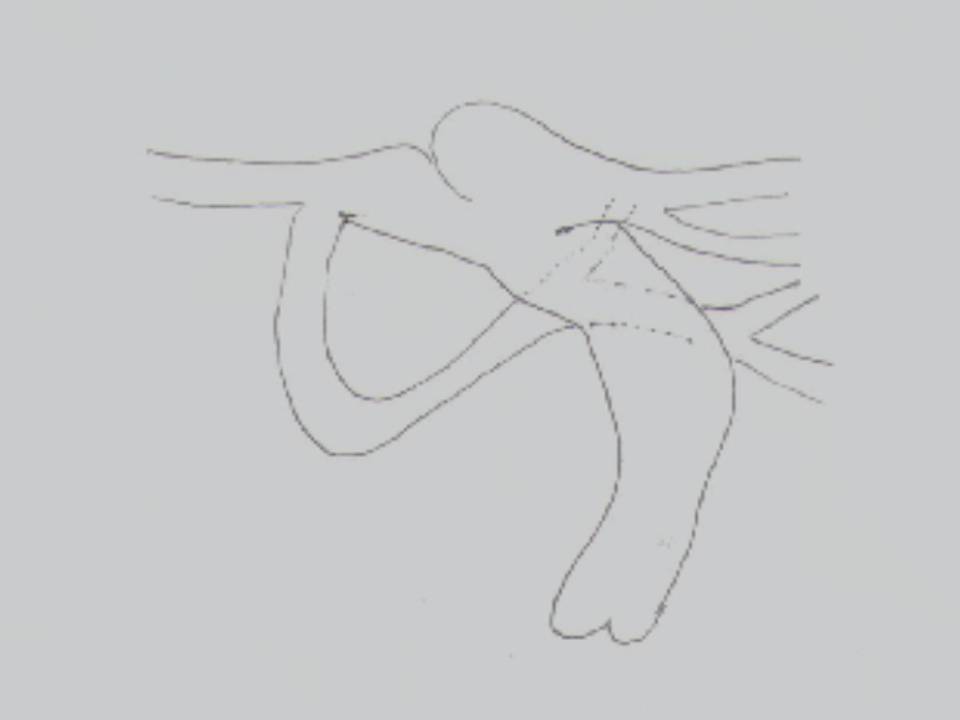
Fig. 6a & Fig. 6b
Figure 6: a. Schematic drawing of the pulmonary artery, its branches and collateral vessel; b. Collateral vessel and its connection to the proximal LPA seen in the transverse orientation.
Discussion:
This patient demonstrates the long term sequelae after repair for Tetralogy of Fallot (pulmonary regurgitation and RV dilatation), as well as the unexpected finding of a residual anomaly (pulmonary collateral vessel from the RPA with a narrow connection to the proximal LPA, and then traversing to and supplying the left lower lobe) and complications of aortic root and valve replacement.
Serial follow-up is crucial in these patients in particular, for assessment of PR and ventricular size and function. CMR is ideal for follow-up as it is able to accurately measure ventricular size and function, which is part of the assessment for determining timing of repair. Various preoperative indexed RVEDV cut-off points have been reported in the literature as the threshold for normalisation after surgery; <170ml/m2 [1], <160ml/m2 [2] and <150ml/m2 [3], but only an indexed RVESV of <90ml/m2 [4] has been shown to be an independent predictor of normalisation after pulmonary valve replacement.
Although this patient is over these limits, pulmonary valve replacement is still a consideration as although RV volumes may not normalise completely, it is expected that there will be some reduction in volumes and therefore improvement in overall function.
There is preferential flow through the RPA origin compared to the LPA accounting perhaps for the much higher flow seen in the relative flow curves. Flow for each branch pulmonary artery was measured at their origins. Although there does not appear to be significant discrete stenosis at the origin of the LPA, the distortion of the origin may contribute to preferential flow thought the unobstructed RPA. Total flow to the LPA, i.e. a combination of that which passes through the LPA origin, and that which is supplied by the collateral vessel, would probably equate net flow through the RPA (flow through the RPA origin-collateral flow to the left lung).
Perspective:
Congenital heart disease is complex and may be heterogenous within disease groups. CMR is ideal for diagnosis and serial follow-up in congenital heart disease as it is excellent for defining anatomy and in assessing function. Currently, it is the only imaging modality that has the ability to measure and quantify RV volumes and function. Most measurements of RV function by other modalities are qualitative at best and not as superior as quantitative analysis by CMR. Furthermore, it is non-radiating, an important consideration for this population of patients who would already have had multiple exposures to radiation. In addition, other diagnoses which are not so easily defined on other imaging modalities can be identified using CMR, as in this case, the finding of a periaortic haematoma, and the collateral artery to the left lung.
An educational purpose in this study is to demonstrate the utility of information other than direct images in the overall assessment of congenital heart disease. In busy centres where there are time constraints for each study, it is not always possible to pick up all anomalies at the time of scanning particularly if it is a more subtle defect. Flow curves are quick and easy to do.
Pulmonary collateral flow from the contralateral pulmonary artery is unusual in Tetralogy of Fallot. Commonly, the collateral vessels originate from the aorta, called major aortopulmonary collaterals (MAPCA). MAPCAs are present in the more severe type of Tetralogy of Fallot, a form that is considered part of the spectrum of pulmonary atresia. Although adult patients have already undergone repair, residual anomalies may be present, and must be assessed for during imaging. An unusual anomaly such as this collateral vessel demonstrates the heterogeneity of congenital heart disease and the need to look out for the unexpected.
A guide to CMR assessment after repair of Tetralogy of Fallot is provided by Tel Geva’s review in JCMR [4].
References:
COTW handling editor: Sylvia Chen
Have your say: What do you think? Latest posts on this topic from the forum







